eDNA water sampling in remote rocky shore environments
Nova Mieszkowska, Dina-Leigh Simons, Helen Hipperson, Tom Webb
Abstract
This is a protocol to collect marine environmental DNA samples from remote rocky intertidal sites by filtering on-site using Sterivex filtering units and a syringe/sealant gun approach. By the end of this protocol, the user will have self-contained and preserved samples in sterviex filters ready for DNA extraction. This protocol has been developed using multiple sources (see references for details).
Before start
Please follow the cleaning regime in a clean lab before sampling. If a clean lab in not available, wipe down all possible surfaces with 10% bleach solution to create a sterile work area.
Steps
Cleaning and packing equipment (pre-sampling)
Put on lab coat and clean non-powdered gloves. Wipe down work area with 10% bleach. If excess dirt is present, scrub surfaces with soap and water before bleaching.
Submerge 1L Nalgene bottles in 20% bleach solution (see solution preparation below) for at least 10-minutes. Be sure to completely expose all surfaces to bleach. If excessive dirt present, scrub with soap and water before submerging in bleach. If previous labelling is present on bottles, use ethanol to wipe off.
Remove 1L Nalgene bottles from bleach and rinse (inside and outside) with deionised water (fill 1/3 of bottle, cap lid, shake, discard water on inside, rinse; repeat 3 times).
Thoroughly dry 1L Nalgene bottles with clean blue roll or air dry on blue roll. Place into separate plastic bag/sterile box for transport to site.
For all other field equipment (e.g. boxes, sealant guns, consumables), wipe down all surfaces with 10% bleach using blue roll.
Count and pack consumables into field box, accounting for the number of desired samples and one negative field control per site. Add one extra set of all consumables to account for breakages, contamination, or unexpected sampling opportunities.
Sensitive consumables (sterivex filters, syringes, combistoppers, and ethanol) should be stored in a secondary smaller box. For ethanol, use a fresh aliquot for each site to minimise contamination.
Transport all equipment in clean plastic bags within field bags (e.g. heavy duty holdall or similar) or sterile boxes to reduce contamination in transit. Avoid supplies from touching the transport bag directly. Use plastic bags to secure waste later.
Purchase sealed bottled drinking water on route to the site for a negative field control.
Sample collection
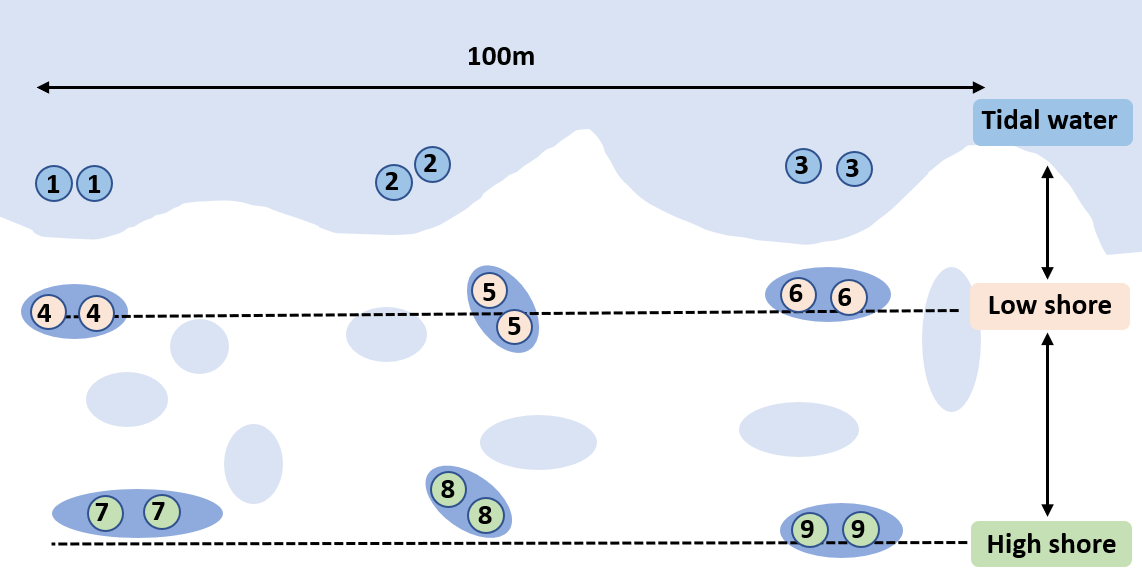
Find an accessible and safe route onto the shore, carrying the bag with 1L Nalgene bottles and field box containing consumables. Find a suitable location to set-up a work station either on the shore or near the car.
Record field metadata (most can be prepared beforehand):
- Site name and grid reference
- County/Area
- Date
- Recorder/s
- Latitude/longitude of access point (e.g., car park)
- Latitude/longitude of centre of survey area (e.g., midshore)
- Exposure scale of the shore
- Weather at the time of the survey, especially the visibility
- Mark site on virtual or physical map
- Tide (incoming or outgoing)

Example of field recording sheet.
Put on non-powdered gloves and remove a 1L Nalgene from the transport bag. Rinse the bottle in the sample water at least three times, ensuring discarded water does not re-enter the sample area.
Fill the bottle halfway (500mL). Proceed to other side of the rockpool and repeat (the bottle should now be full). Secure the lid.

Optional: Take an image of the rockpool sampled with one-metre measuring ruler for scale. Record depth, temperature, pH and salinity using a two-point measurement system (average when entering the data).
Change gloves.
Repeat steps 12-17 for two more rockpools at same shore height (i.e. lowshore), three more rockpools at different shore height (i.e. highshore), and three open water samples (image and depth measurement not required for open water samples).
Sample filtration
Partially tear open the sterivex filter packaging to expose the filter inlet (female), as instructed on the
packaging. Tear open the 60mL syringe packaging and remove the plunger. Twist the Sterivex inlet into the syringe.
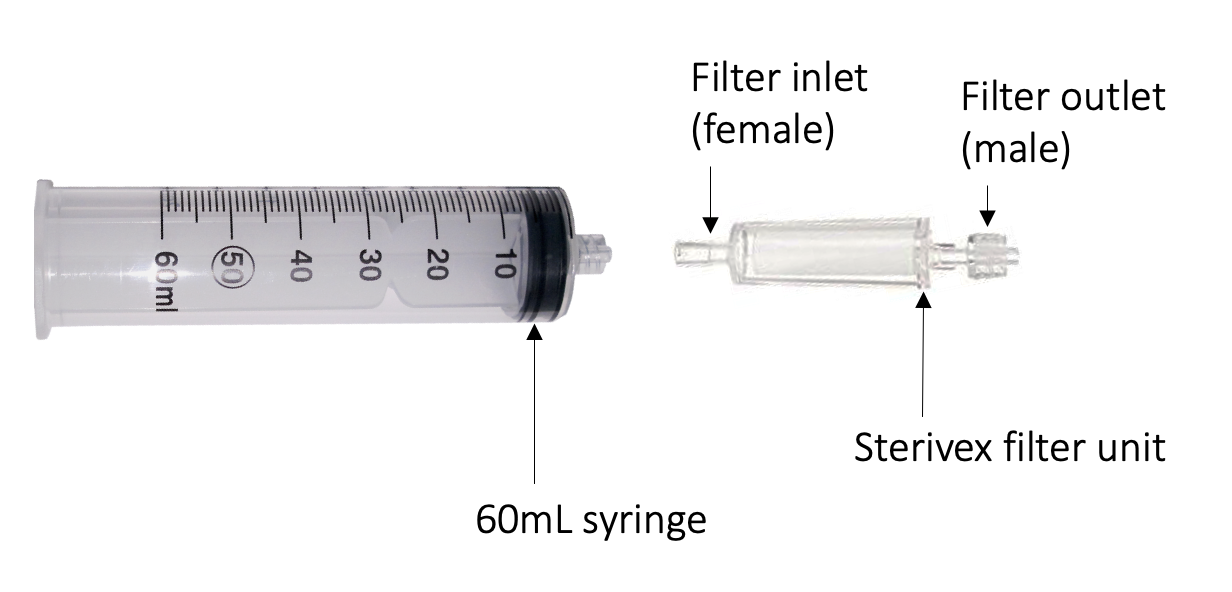
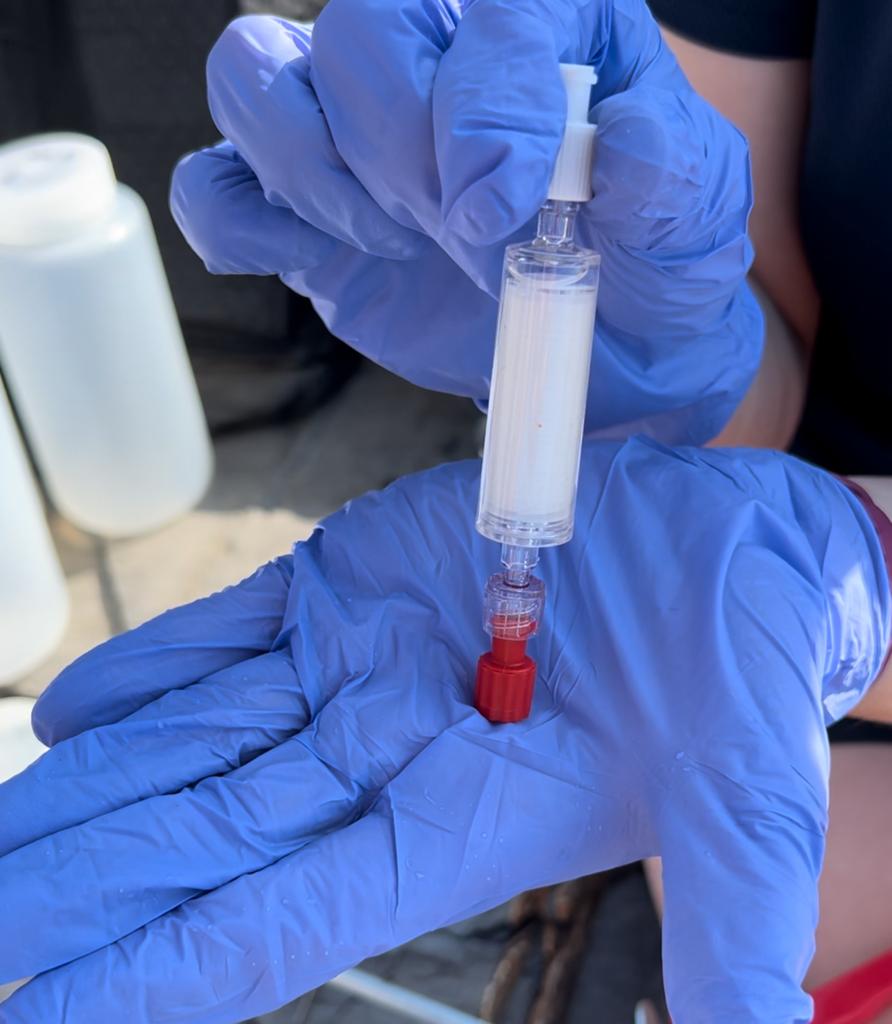
Fill the syringe with 60mL of water sample and place the plunger back into the end.
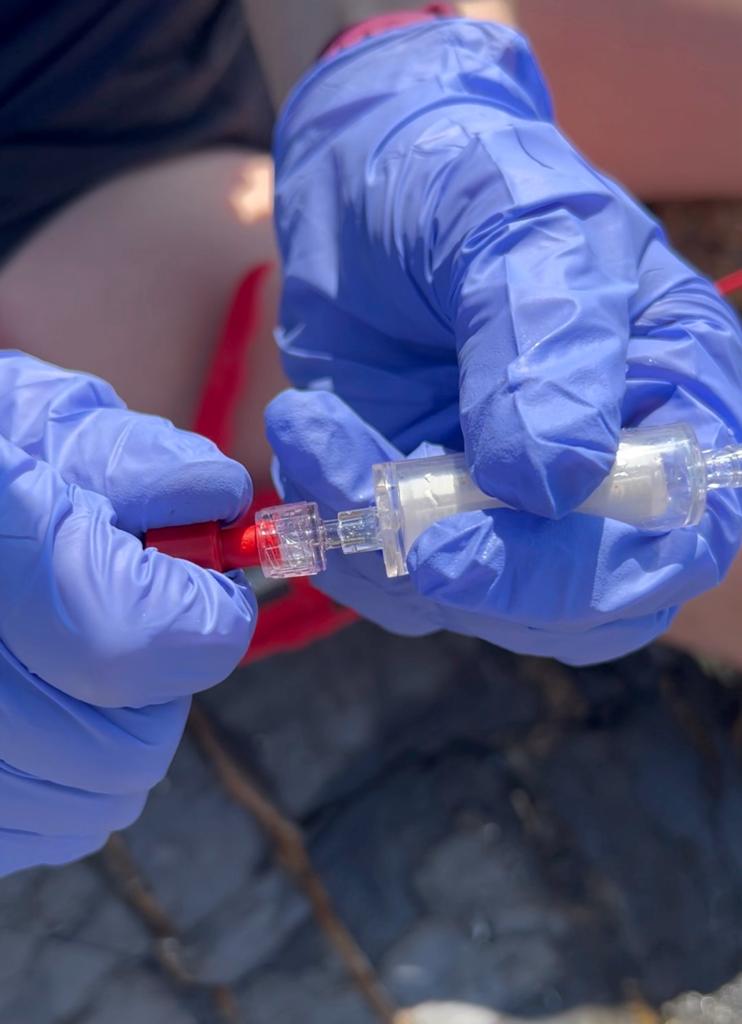
Insert the syringe and filter into the sealant gun and fire the gun until the liquid has gone through, making sure to pump waste water away from equipment. Once all the water has passed through, detach the filter, pull the syringe plunger out, and reattach filter.
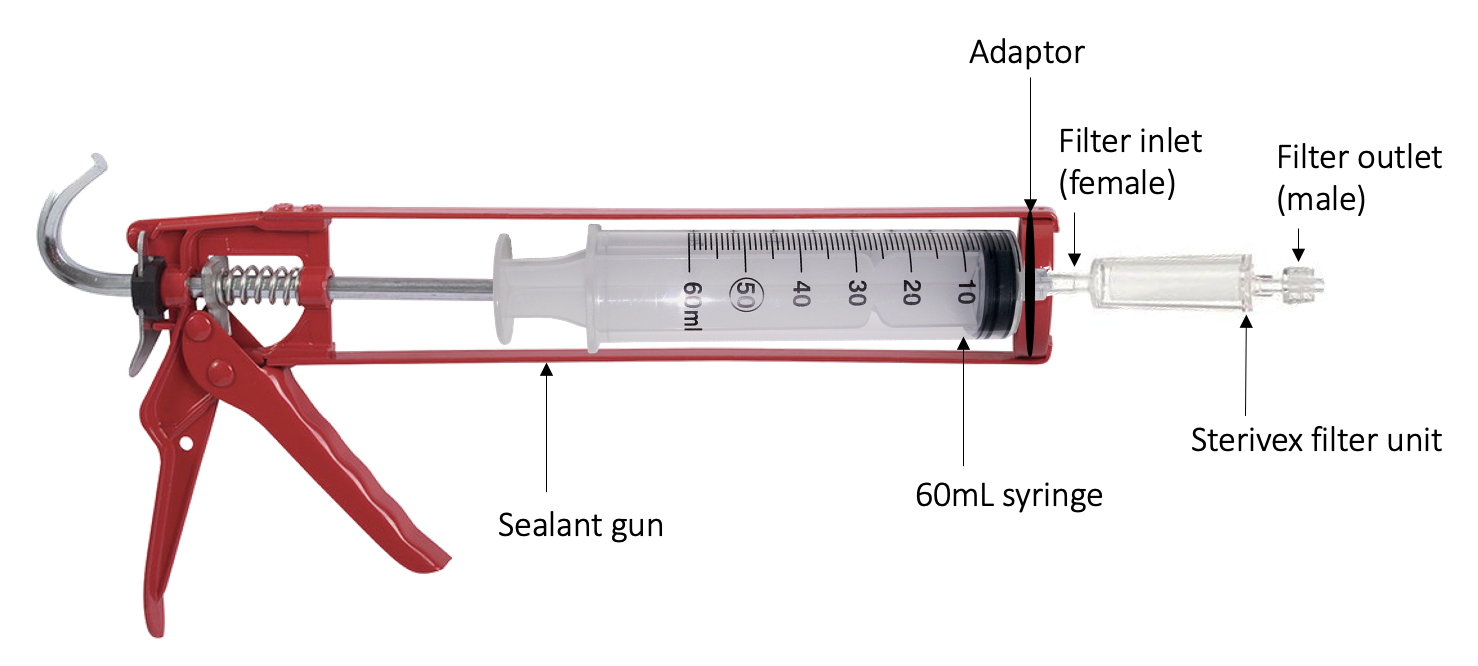

Repeat steps 21-22 until you have filtered all the water in the grab bottle.
Once the desired volume has been reached, detach the filter. Then, remove and reinsert the plunger to fill the syringe with air, and reattached the filter. Press the syringe down while attached to the filter to drain remaining water from inside the filter.
Draw up2mL preservation solution from the ethanol aliquot using 3mL syringe.
Attach 3mL syringe filled with 2mL preservation solution to the inlet (female) and add to filter.

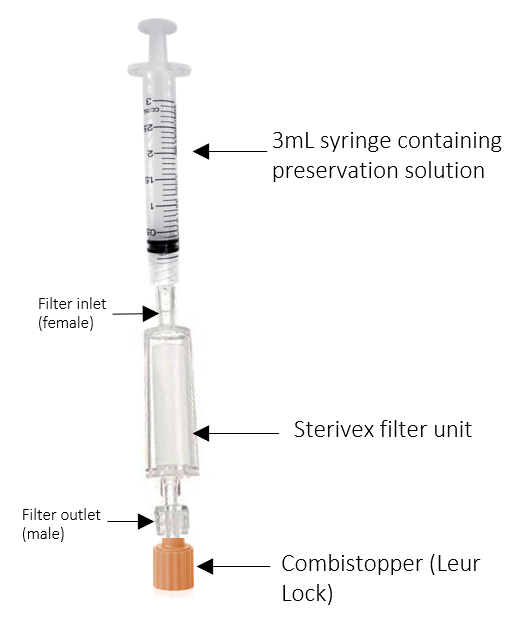
Repeat steps 20-30 for all collected water samples.
For the negative control, rinse a clean grab bottle at least three times using the sealed bottled water. Pour 200mL of the bottled water into the grab bottle and secure the lid. Repeat steps 20-30. The control should be filtered and preserved using the same equipment and procedures as the samples.
Post-sampling checks
Before leaving the site, have one last walk around the sample site to confirm metadata details. Check that all equipment and waste has removed from the shore.
All filters should be transferred to a -20°C spark proof laboratory freezer as soon as possible. Filters can be stored unfrozen for up to one month, in a fridge or on ice packs if possible.
All equipment must be cleaned and packed away before next use (following the cleaning and packing instructions above). It is important to clean thoroughly between sites.