WATER PRODUCTION FOR AWARE (Parasite)
Elvira Abollo, Santiago Pascual
water sampling
water processing
water analysis
waste water treatment
advanced tertiary treatment SOP
Abstract
The protocol summarises the procedures used for analytical control. The protocol describes the Standard Operating Procedure (SOP) for the optimization of advanced tertiary treatment of water, based on a comprehensive quality and risk assessment.
Steps
Parasite
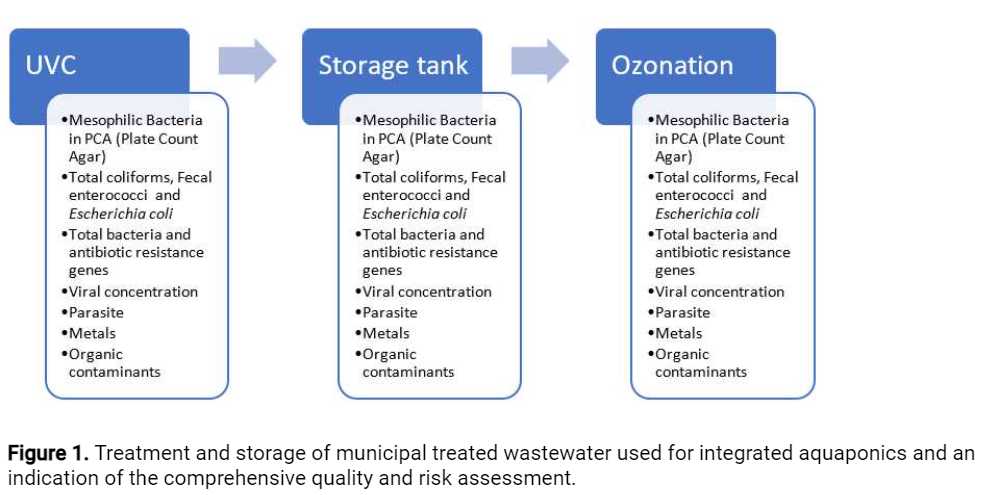
The water production for AWARE main activities includes three stages – disinfection by ultraviolet C radiation (UVC), storage for12h 0m 0s-24h 0m 0s(according to water load and season) and ozonation. The water quality is monitored at these three stages, for the parameters indicated in Figure 1 below.
Sampling, Processing, and Analyses
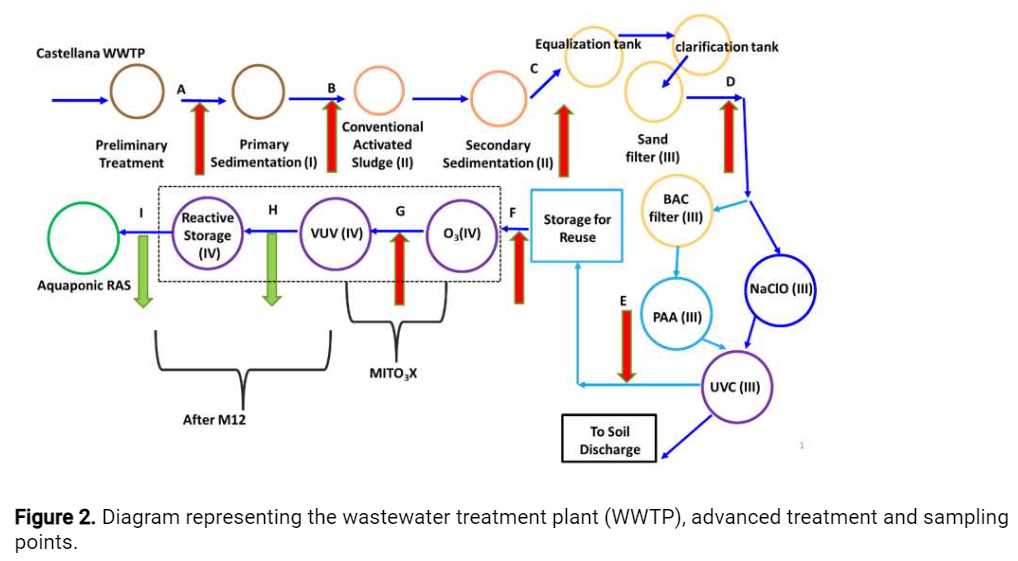
Water samples are collected (see Figure 2) and processed within a6h 0m 0sinterval, before being shipped for the partner responsible for the analyses (Table 1). In case no processing is needed, samples are frozen and stored at-80°Cwithin3h 0m 0s.
For each sampling event, the date, day of the week and hour; the temperature and rain. Sampling points, indicated in Figure 2 were designated from A to I:
-
Influent of primary treatment (A)
-
Influent of biological treatment (activated sludge) (B)
-
Treated secondary effluent (C)
-
Sand filter effluent (D)
-
UVC effluent (E)
-
Storage for reuse tank effluent (F)
-
Ozonation effluent (1 dose, e.g.,
5mgO3) - MITO3X technology - (G) -
Effluent of the vacuum UV oxidation (VUV) (H)
-
Effluent of reactive storage / Influent of the recirculation aquaculture system (RAS) (I)
Methods: Processing of Water samples - The section below summarises the procedures used for analytical control – detailed protocols are annexed to this protocol.
Parasites: Parasites:
Analysis: Detection of parasites.
Filtration:
3Lof water were filtered through 0.45 µm pore size diameter Nitrocellulose / polycarbonate sterile membranes, using a membrane filter holder (47 mm diameter) apparatus. For every approx.250mLof water, a new sterile membrane was used to avoid clogging of membranes. Filters corresponding to each litre of water were separated, inserted into well-sealed tubes (50mL stored a-80°Cand then transported in dry ice.
Centrifugation:
(1L) of water was concentrated by centrifugation at2.500gfor0h 15m 0sususing sterilized tubes. Pellets obtained were resuspended using of1mLof10formaldehyde solution and maintained for24h 0m 0s. Pellets resuspended in formaldehyde solution were centrifugated at2.500gfor , the supernatant was discarded and all pellets were pooled in1mLof70 ethanol.
DNA Isolation, Amplification and sequencing:
DNA extraction was carried out using a commercially available kit (Powerwater kit, Qiagen) in accordance with the manufacturer's guidelines. The V4 and V9 of the 18S rRNA gene hypervariable regions of the eukaryote 18S rRNA gene were amplified using universal primers. Subsequently, PCR products were purified and subjected to external sequencing using Illumina MiSeq platform 300bp paired-end sequencing.
Bioinformatic analysis:
Raw sequence data were processed using QIIME2 software 2023.9. Pairedend reads were subjected to quality filtering (FastQC) and denoising (DADA2 plugin). Subsequently, the taxonomic assignment was conducted using the reference database SILVA 18S release 138 with a clustering threshold of99similarity. Alpha rarefaction analysis, Shannon diversity index, richness estimation, and relative abundance calculations were performed using the Vegan R package to assess microbial community diversity and composition particularly focusing on the detection of genetic material from potential pathogens.
Observations: Samples were filtered within12h 0m 0safter collection the filtering membranes were immediately frozen and stored a-80°Ctill shipping in dry ice to the respective partner for further analysis.
Parameters framed by Legal and Regulatory Requirements:
Using the EU Drinking Water Directive:
Mesophilic Bacteria in PCA (Plate Count Agar) – 0 CFU/100mL
Total coliforms and Escherichia coli –Number /100mL(0 MPN/100mL)
Fecal enterococci –Number /100mL(0 MPN/100mL)
Viral concentration - There are no legal requirements for viruses. They are not included in any regulation now.
Parasite - EU legislation (2020/741)
Metals - DIRECTIVE 2008/105/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2008 on environmental quality standards in the field of water policy
Organic contaminants - DIRECTIVE 2008/105/EC OF THE EUROPEAN PARLIAMENT AND THE
COUNCIL of 16 December 2008 on environmental quality standards in the field of water policy.

