Single nuclei isolation from frozen human adipose tissue for 10x Genomics multiome sequencing
Lynn M Geletka, Clarissa Streider-Barboza, Robert W. O"Rourke, Carey Lumeng
Abstract
Here we present a modified version of a 10x Genomics demonstrated protocol that we adapted for the isolation of nuclei from human adipose tissue. Single-nuclei are isolated from 1-1.2g frozen surgery-derived biopsies of human omental or subcutaneous abdominal adipose tissue and stained with 7-AAD for FACS sorting. The sorted nuclei are permeabilized and final resuspension takes place in Nuclei Buffer supplied in the 10x Genomics Chromium Next GEM Single Cell Multiome ATAC + Gene Expression Reagent bundle. This protocol does not describe library construction or sequencing.
Before start
Read through the entire protocol before beginning. This protocol has been developed using a two-step homogenization process utilizing a porcelain mortar with a porcelain pestle, and a conical glass grinder tube with a PTFE pestle. Substitutions for this equipment will require optimization.
During the homogenization process, lipids will aggregate and form solid lumps while on ice. They will also smear along the side of the conical glass grinder tube. Since they can interfere with downstream processing and clog the strainers, we transfer only the liquid portion of the homogenate to the 100um strainer using a 2mL pipet with a large bore.
We strongly suggest testing the protocol using buffers prepared without RNase inhibitor to observe the number of nuclei resulting from the homogenization process, and the expected volume of nuclei sample coming off the FACS sorter, before proceeding with any experimental samples.
Precaution: for the ATAC-Seq portion of the 10x Genomics multiome analysis process, you will need to use the proprietary 20x Nuclei Buffer supplied in the "Chromium Next GEM Single Cell Multiome ATAC + Gene Expression Reagent Bundle."
Steps
Buffer Preparation
Prepare all the buffers listed in the tables below. Any buffers that contain RNase Inhibitor must be prepared the day of the nuclei isolation. Buffers without RNase Inhibitor may be prepared a day ahead.
NP40 Lysis Buffer: (A)
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Ingredient | Source | Ref. No. | Stock Conc. | Final Conc. | Volume for 10mL |
| Tris-HCl (pH 7.4) | Sigma | T2194-100ML | 1 M | 10 mM | 100uL |
| NaCl | Promega | V4221 | 5 M | 10 mM | 20uL |
| MgCl2 | Sigma | M1028-100mL | 1 M | 3mM | 30uL |
| Nonidet P40 Substitute | G-Biosciences | DG001 | 10% | 0.1% | 100uL |
| DTT | Sigma | 43816-10mL | 1000 mM | 1 mM | 10uL |
| Nuclease-free water | Invitrogen | 10977 | 9490uL | ||
| RNase inhibitor | Sigma | 3335402001 | 40 U/uL | 1U/uL | 250uL |
DPBS/1% BSA/1U/uL RNase Inhibitor Buffer:
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Ingredient | Source | Ref. No. | Stock Conc. | Final Conc. | Volume for 3mL |
| DPBS-/- | Gibco | 14190-144 | 1X | 2625uL | |
| BSA* | Sigma | A7030-100G | 10% | 1% | 300uL |
| RNase inhibitor | Sigma | 3335402001 | 40 U/uL | 1U/uL | 75uL |
- Bovine Serum Albumin powder dissolved in DPBS-/- and filter sterilized.
Lysis Buffer (to prepare 0.1 X Lysis Buffer): (B)
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Ingredient | Source | Ref. No. | Stock Conc. | Final Conc. | Volume for 0.5mL |
| Tris-HCl (pH 7.4) | Sigma | T2194-100ML | 1 M | 10 mM | 5uL |
| NaCl | Promega | V4221 | 5 M | 10 mM | 1uL |
| MgCl2 | Sigma | M1028-100mL | 1 M | 3mM | 1.5uL |
| Tween-20 | Bio-Rad | 1610781 | 10% | 0.1% | 5uL |
| Nonidet P40 Substitute | G-Biosciences | DG001 | 10% | 0.1% | 5uL |
| Digitonin | Invitrogen | BN20061 | 5% | 0.01% | 1uL |
| BSA | Sigma | A7030-100G | 10% | 1% | 50uL |
| DTT | Sigma | 43816-10mL | 1000 mM | 1 mM | 0.5uL |
| Nuclease-free water | Invitrogen | 10977 | 418.5uL | ||
| RNase inhibitor | Sigma | 3335402001 | 40 U/uL | 1U/uL | 12.5uL |
Lysis Dilution Buffer (to prepare 0.1 X Lysis Buffer): (C)
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Ingredient | Source | Ref. No. | Stock Conc. | Final Conc. | Volume for 1mL |
| Tris-HCl (pH 7.4) | Sigma | T2194-100ML | 1 M | 10 mM | 10uL |
| NaCl | Promega | V4221 | 5 M | 10 mM | 2uL |
| MgCl2 | Sigma | M1028-100mL | 1 M | 3mM | 3uL |
| BSA | Sigma | A7030-100G | 10% | 1% | 100uL |
| DTT | Sigma | 43816-10mL | 1000 mM | 1 mM | 1uL |
| Nuclease-free water | Invitrogen | 10977 | 859uL | ||
| RNase inhibitor | Sigma | 3335402001 | 40 U/uL | 1U/uL | 25uL |
0.1 X Lysis Buffer:
| A | B | C | D | E |
|---|---|---|---|---|
| Ingredient | Source | Stock Conc. | Final Conc. | Volume for 1mL |
| 1 X Lysis Buffer (B) | Prepared in lab | 1 X | 0.1 X | 100uL |
| Lysis Dilution Buffer (C) | Prepared in lab | 900uL |
Wash Buffer: (D)
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Ingredient | Source | Ref. No. | Stock Conc. | Final Conc. | Volume for 2mL |
| Tris-HCl (pH 7.4) | Sigma | T2194-100ML | 1 M | 10 mM | 20uL |
| NaCl | Promega | V4221 | 5 M | 10 mM | 4uL |
| MgCl2 | Sigma | M1028-100mL | 1 M | 3mM | 6uL |
| BSA | Sigma | A7030-100G | 10% | 1% | 200uL |
| Tween-20 | Bio-Rad | 1610781 | 10% | 0.1% | 20uL |
| DTT | Sigma | 43816-10mL | 1000 mM | 1 mM | 2uL |
| Nuclease-free water | Invitrogen | 10977 | 1698uL | ||
| RNase inhibitor | Sigma | 3335402001 | 40 U/uL | 1U/uL | 50uL |
2X Diluted Nuclei Isolation Buffer:
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Ingredient | Source | Ref. No. | Stock Conc. | Final Conc. | Volume for 0.2mL |
| Nuclei Buffer* | 10X Genomics | 2000153/ 2000207 | 20x | 2x | 20uL |
| DTT | Sigma | 43816-10mL | 1000 mM | 2 mM | 0.4uL** |
| RNase inhibitor | Sigma | 3335402001 | 40 U/uL | 2 U/uL | 10uL |
| Nuclease-free water | Invitrogen | 10977 | 169.6uL |
- Nuclei Buffer supplied in the 10x Genomics Chromium Next GEM Single Cell Multiome ATAC + Gene Expression Reagent bundle.** Can dilute DTT 1:5 in water and use 2uL. Reduce water volume to 168uL.
Nuclei Isolation
Transfer frozen adipose tissue to a 300mL porcelain mortar pre-chilled and filled with liquid nitrogen. Press/tap on the frozen tissue with a pre-chilled porcelain pestle to pulverize it. A little grinding may be necessary.
Transfer the pulverized tissue to an ice-cold 15mL conical glass grinder tube, (using a pre-chilled metal spatula), just after the liquid nitrogen disappears.
Immediately add 2mL of cold NP40 Lysis Buffer to the tissue.
Homogenize the tissue/cells in the grinder tube with a cold conical PTFE pestle. Use twisting strokes with the pestle. The number will be determined by the appearance of tissue, (e.g.,12 strokes).
Tips:
• keep on ice all the time, (the conical grinder tube should be immersed in ice)
• use gentle strokes, (with pressure if necessary)
• avoid foaming
• add more NP40 Lysis Buffer, if necessary, (not more than 900uL)
• use the number of strokes necessary to reach the bottom of the tube with the pestle
Using a 2mL pipet with a wide bore, transfer the liquid portion of the homogenate only to a 100um cell strainer (Biologix, Ref. No. 15-1100, or other) pre-wetted with 150uL of NP40 Lysis Buffer and placed on a 50mL conical polypropylene centrifuge tube.
Wash the contents of the conical glass grinder tube with 500uL of NP40 Lysis Buffer and transfer this to the strainer as well.
Wash the strainer with 500uL of NP40 Lysis Buffer.
Pass the filtered homogenate through a 40um cell strainer (Falcon, Ref. No. 352340, or other) pre-wetted with 150uL of NP40 Lysis Buffer into another 50mL conical polypropylene centrifuge tube. Wash the strainer with 500uL of NP40 Lysis Buffer.
Transfer the contents of the 50mL tube to a 15mL polypropylene centrifuge tube.
Centrifuge at 500 rcf for 5 min at 4℃ in a swinging-bucket rotor.
Carefully remove any lipid layer with a plastic transfer pipet, then carefully remove the supernatent down to about 50uL using a 1mL pipet tip.
Add 1mL of DPBS/1% BSA/1U/uL RNase Inhibitor, but DO NOT mix.
Incubate on ice for 5 min.
Pipette mix to resuspend pellet (5x). Leave the sample in the 15mL centrifuge tube.
Centrifuge at 500 rcf for 5 min at 4℃.
Remove the supernatent.
Resuspend in 400uL of DPBS/1% BSA/1U/uL RNase Inhibitor and pipet to mix (5x). Keep on ice.
Repeat steps 2-18 for additional samples.
Nuclei Sorting (protocol for each sample)
Transfer 25uL of unstained nuclei sample to a 5mL polystyrene, round-bottom (FACS) tube (Corning Falcon, Ref. No. 352008) containing 250uL of DPBS/1% BSA to reserve unstained. This only needs to be performed for one sample.
Transfer the rest of the nuclei prep, plus 100uL of DPBS/1% BSA/1U/uL RNase Inhibitor used to rinse the 15mL tube, to another 5mL FACS tube. Add 7AAD Ready-Made Solution (Sigma-Aldrich, Ref. No. SML1633) to this nuclei sample, (1uL 7AAD per 100uL sample). 7-AAD is excited with a 488 laser and emission is detected around 647nm.
Incubate for at least 5 minutes on ice with light exclusion.
Fill a 5mL FACS collection tube with DPBS/5% BSA and incubate for 5 minutes to coat the collection tubes with protein.
Remove the DPBS/5% BSA from the collection tube and rinse with PBS/1% BSA.
Pipet enough DPBS/10% BSA and 40U/uL RNase Inhibitor into the collection tube to achieve a final concentration of 1% BSA and 1U/uL RNase Inhibitor after the addition of the sorted nuclei. Volumes should be determined empirically through testing of FACS.
Proceed to FACS machine for nuclei sorting. Use a 100um nozzle. See the gating strategy below.
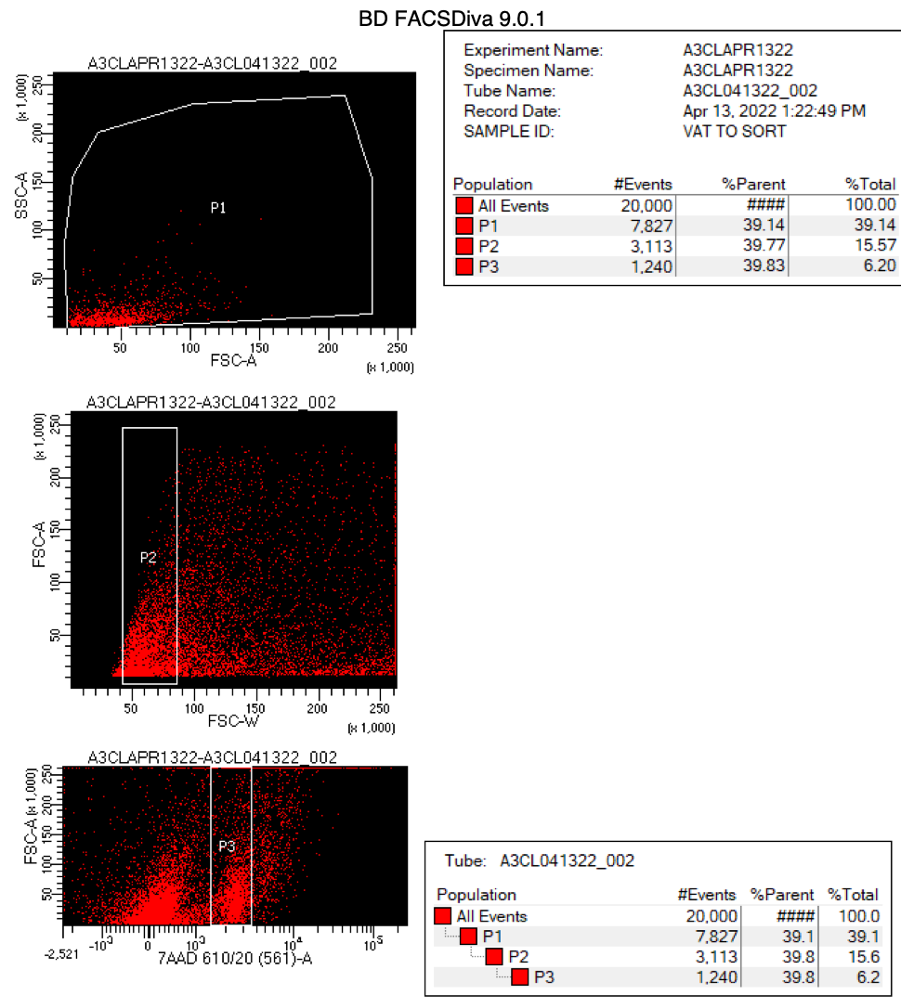
Nuclei Permeabilization (protocol for each sample)
Transfer sorted nuclei to a 15mL polypropylene tube and centrifuge at 500 rcf for 5 min at 4℃.
Remove the supernatent without disrupting the nuclei pellet down to about 25uL. The pellet will not be visible.
Resuspend the pellet in 100uL of 0.1X Lysis Buffer and pipet to mix (5x).
Incubate for 2 min on ice.
Add 1mL of Wash Buffer and pipette to mix (5x).
Centrifuge at 500 rcf for 5 min at 4℃.
Remove the supernatent without disrupting the nuclei pellet down to about 25uL.
Resuspend the pellet in an equal volume of 2X Diluted Nuclei Isolation Buffer. Pipet to mix (5x). The nuclei are now ready for counting and proceeding to the CG000338 Chromium Next GEM Multiome ATAC + GEX User Guide (Version F).
Expected Results
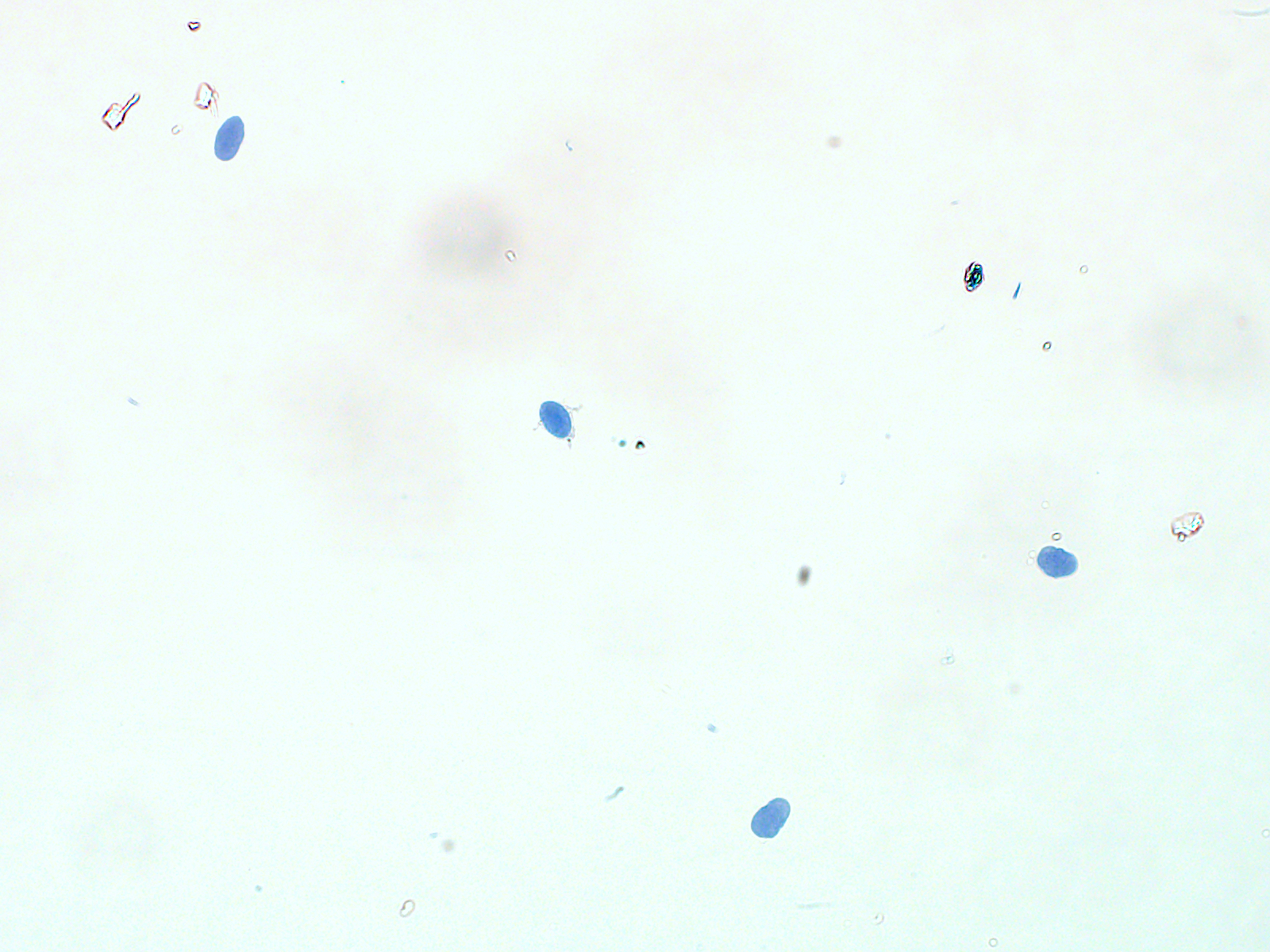
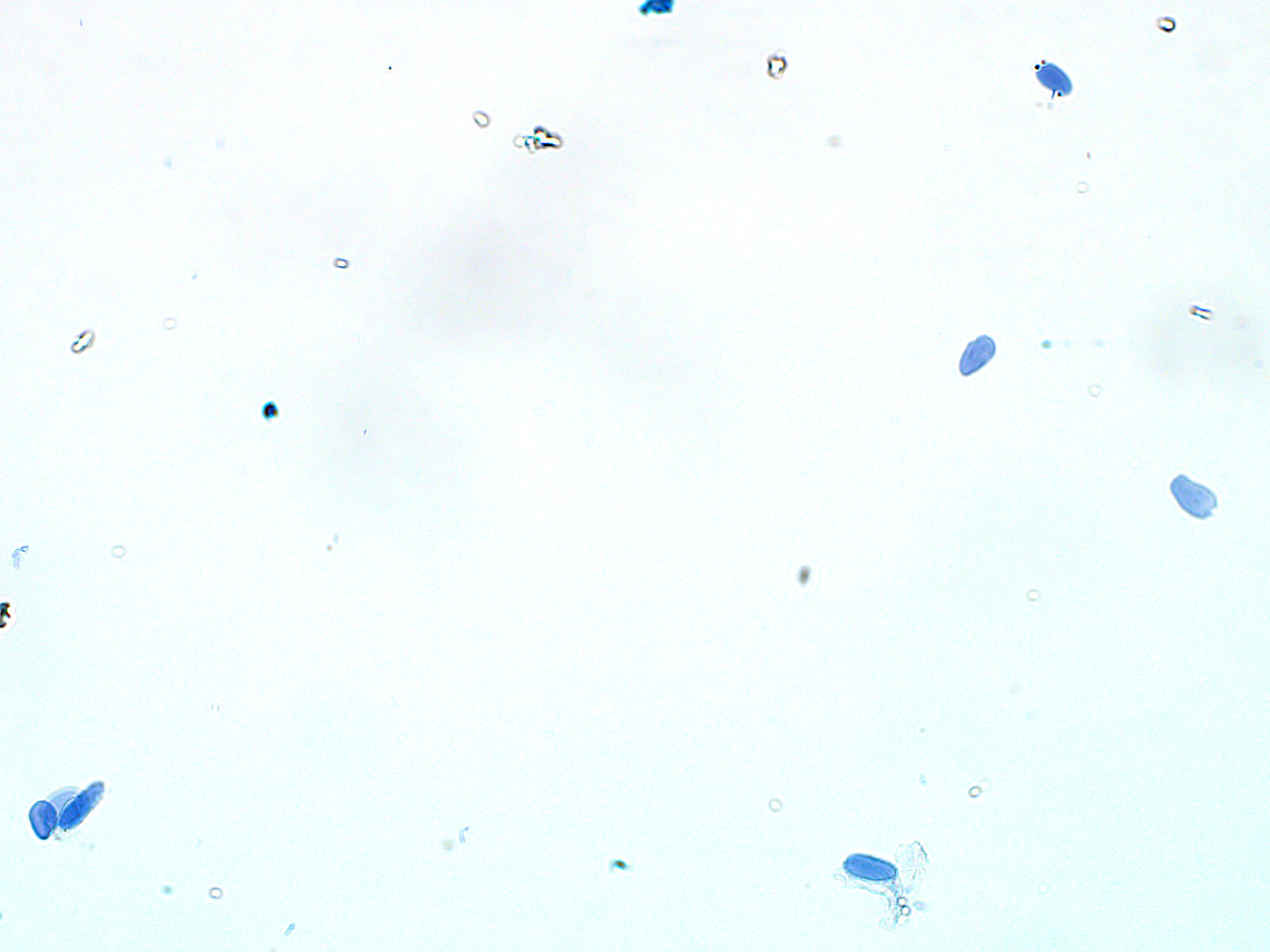
After sorting and permeabilizing the nuclei, they should appear to be mostly single and intact. We obtained about 1.4 x 105 nuclei/gram of omental adipose tissue, and 7.4 x 104 nuclei/gram of subcutaneous abdominal adipose tissue.