Human Neuronal Nucleus Isolation for Single-Nucleus Transcriptomic Profiling (10x Genomics)
Satoshi Ishishita, Allan-Hermann Pool
Abstract
Protocol generating suspensions of neuronal nuclei (NeuN+) from human central nervous system tissue for single-nucleus transcriptomics.
Steps
Equipment and Reagents
Equipment
- Kimble Dounce Kontes tissue-grinder set (DWK 885300-0000)
- 50 ml Oakridge tubes (#0556214D)
- 15 mL Falcon tubes (Fisher #352097)
- 50 mL Falcon tubes (Fisher #352070)
- 1.5mL LoBind Eppendorf Tubes
- 70-micron Corning Cell Strainer (#431751)
- Fire polished glass Pasteur pipettes (VWR #14672-380, polished in an open gas flame down to ~600 micron, 300 micron and 150 micron tip opening sizes)
Reagents
- Roche Protector RNase Inhibitor (Millipore Sigma RNAINH-RO)
- BioLegend anti-NeuN antibody (#608452, for labeling neuronal nuclei)
- Alexa Fluor 647 Microscale Protein Labeling Kit (ThermoFisher #A30009)
- 1 mg/ml 7-AAD (nuclear labeling)
- 1M DTT (dithiothreitol, prepare fresh every couple of months and store at -20°C)
- Ultrapure RNA-se free/ DNA-se free water
Solutions
NMDG-Hepes-ACSF
- NMDG (93 mM)
- KCl (2.5 mM)
- NaH2PO4(1.2 mM)
- NaHCO3 (30 mM)
- HEPES (20 mM)
- Glucose (25 mM)
Bring pH to between 7.3 - 7.4 with 10N HCl and filter sterilize (good for 2 weeks at 4°C).
On the morning of tissue preparation add the following components (final concentration):
-
Na-Ascorbate (5 mM)
-
Thiourea (2 mM)
-
Na-pyruvate (3 mM)
-
MgSO4 (10 mM, prepare 2M stock that is good for 6-months at 4°C)
-
CaCl2 (1 mM, prepare 2M stock that is good for 6-months at 4°C)
-
Kynurenic acid Na-salt (1 mM)
Nuclear Buffer
- Sucrose (320 mM)
- Tris-HCl (pH=7.4) (10 mM)
- MgCl2 (3 mM)
- NaCl (10 mM)
- BSA (RNAse free) (0.50%)
- Kollidon VA64 (1 %)
- Ultrapure water, fill to 50 mL and 0.22 micron filter sterilize.
Morning of run:
- DTT (dithiothreitol, 1 mM)
- Roche Protector RNAse Inhibitor (0.1 U/uL)
Lysis Buffer
- Nuclear buffer
- Triton-X100 (0.1%)
1M Sucrose Cushion
Sucrose (1 M)
Tris-HCl (pH=7.4) (10 mM)
MgCl2 (3 mM)
NaCl (10 mM)
BSA (nuclease free) (0.50%)
Kollidon VA64 (1%)
Water (molecular biology grade) Fill to 50 mL
Do NOT Filter sterilize!
Resuspension Buffer
- Sucrose (320 mM)
- Tris-HCl (pH=7.4) (10 mM)
- MgCl2 (1 mM)
- NaCl (10 mM)
- BSA (RNAse free) (0.50%)
- Ultrapure water, fill to 50 mL and 0.22 micron filter sterilize.
Morning of run:
- DTT (dithiothreitol, 1 mM)
- Roche Protector RNAse Inhibitor (0.1 U/uL)
Protocol
1. Prepare solutions and equipment
-
Prepare 50 mL of NMDG-HEPES-ACSF from pre-prepared stock by adding (Na-Ascorbate, Thiourea, Na-pyruvate, MgSO4, CaCl2 and Kynurenic acid Na-salt) and place on ice.
-
Prepare Nuclear Buffer (add DTT and RNA-se inhibitor to preprepared solution) and place on ice.
-
Prepare Lysis Buffer from Nuclear Buffer (add Triton-X100 to 0.1% of final volume) and pipette 0.75 mL into a Kontes tissue grinder.
-
Prepare 1M sucrose cushion (add DTT and RNA-se inhibitor to preprepared solution) and place on ice.
-
Pre-cool centrifuge to 4°C.
-
Place 100 mm dissection dish into a 150 mm dish with dry ice.
2. Dissect out tissue
Place snap frozen brain tissue into 100 mm tissue culture dissection dish on a layer of dry ice in a larger 150 mm dish. Microdissect out desired tissue parts and cut into small 1.5 mm3 cubicles. Drop the latter into ice-cold NMDG-Hepes-ACSF in 1.5 mL collection tubes on ice.
3. Generate nuclear suspension
-
Transfer tissue pieces into the Lysis Buffer in the Kontes tissue grinder.
-
Apply 5 strokes with the loose pestle followed by 15 strokes with the tight pestle.
-
Place a 70-micron cell strainer on a 50 mL Falcon tube and pre-wet with 500 μL of Nuclear Buffer.
-
Add 250 μL of Nuclear Buffer to the tissue grinder.
-
Mix nuclear suspension in tissue grinder twice with a 600-micron fire polished glass capillary and transfer through the cell strainer.
-
Wash tissue grinder with 750 μL Nuclear Buffer and transfer again through the cell strainer.
-
Wash cell strainer with final 750 μL Nuclear Buffer.
4. Spin nuclei down and resuspend in fresh Nuclear Buffer
-
Spin nuclei down for 5 min at 500g at 4°C in a spin-out rotor.
-
Remove supernatant and resuspend in fresh 3 mL Nuclear Buffer.
5. Purify nuclei with a sucrose cushion centrifugation
-
Transfer 12 mL of sucrose cushion into a 50 mL Oakridge tube.
-
Gently layer the nuclear suspension from the previous step on the sucrose cushion (avoid mixing of the layers).
-
Centrifuge the tubes at 3200g at 4°C for 20 minutes in a spin-out rotor.
-
After centrifugation, pour out the supernatant by decanting in one smooth motion and drying out the neck of the Oakridge tube with a Kimwipe.
-
Resuspend the nuclear pellet in 300 μL of ice-cold Nuclear Buffer and mix gently with a 300 micron fire polished Pasteur pipette.
-
Transfer purified nuclear suspension to a new 15 mL tube on ice.
6. Stain nuclei for FACS sorting
-
Add 3 μL of anti-NeuN-Alexa-647 Ab and 3 μL of 7-AAD to the nuclear suspension.
-
Incubate the nuclear suspension at 4°C (not on ice!; keep dark as 7-AAD is light sensitive).
-
Bring nuclear suspension volume to 3 mL with Nuclear Buffer.
-
Spin nuclei down at 500g for 5 min at 4°C in a spin-out rotor.
-
Remove most of supernatant and resuspend nuclei in 3 mL of Nuclear Buffer.
-
Place suspension at 4°C for 5 min.
-
Spin nuclei down at 500g for 5 min at 4°C.
-
Remove supernatant and resuspend nuclei in 0.5 mL of Nuclear Buffer.
-
Gently triturate with 150 micron glass Pasteur pipette to declump any nuclei.
-
Add 1 mL of Nuclear Buffer and take to FACS sorting.
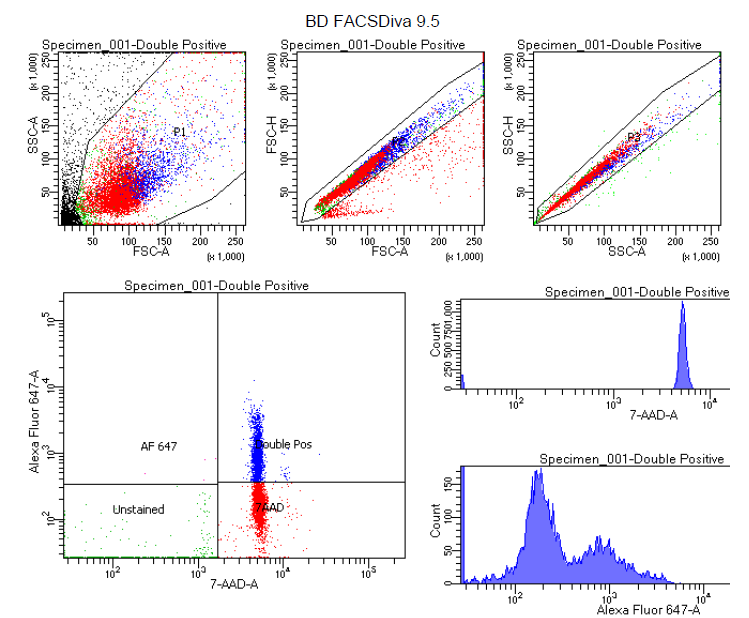
7. FACS sorting
-
Sort at low pressures (4 or 5 PSI on BD sorters).
-
Select for 7-AAD and Alexa 647 double-positive nuclei (note - perform compensation with single-stains of dyes before the actual profiling run to get clear separation between double-positive neuronal nuclei and single-positive non-neuronal nuclei).
8. Concentrate nuclei
-
Spin FACS sorted nuclei down at 500g for 5 min at 4°C in a spin-out rotor and gently resuspend in desired buffer for 10x profiling (e.g. Resuspension Buffer above). Note 1 - post-FACS centrifugation is ok with human neuronal nuclei but not recommended with post-FACS mouse nuclei.
-
During optimization stage, visualize nuclei with a 40x objective in brightfield and make sure resulting nuclei have predominantly (~85%+) round intact nuclear membranes.
-
Optional: gently pipette the suspension with 150 micron fire polished Pasteur pipette to get rid of any remaining nuclear clumps.
-
Proceed to 10x Genomics or other profiling.

