Simple and fast technique for separating human mononuclear cells from small amounts of blood and buffy coat with high cell yield
Sudhir Bhatia, Gudrun Baersch
Mononuclear cell separation
Magnetic beads
Genekam MNC isolator
Cell therapies
CAR-T
ELISA
B cells
T cells
CD4
CD8
Disclaimer
Genekam Biotechnology AG
Duissernstr. 65a
47058 Duisburg
Germany
Abstract
We are again describing a rare and very simple protocol here:
The applications for human mononuclear cells (MNCs) are increasing, as these cells can be used for various tests, cell therapies and the development of human therapeutic antibodies. Density gradient isolation is still used today to separate MNCs. Diluted blood or buffy coat is added to the density gradient liquid, which is then centrifuged to obtain the different fractions. The white layer contains the MNCs, which are carefully pipetted out and washed to obtain the cells. The disadvantage of this laborious procedure is that blood and density gradient fluid may mix, which can lead to wastage of the valuable blood sample.
We have developed a simple procedure in which the user waits a few minutes after mixing the blood sample in the Quick MNC isolator in a tube. The sample is then washed two or three times with PBS and centrifuged to obtain a pellet. Although small amounts of red blood cells occasionally remain in the pellet, they have no effect on the cells in the assay, e.g. when separating with magnetic beads, or on further experiments with isolated MNC cells. A high cell yield can be achieved with a single milliliter of blood or buffy coat. This MNC isolator method is an extremely fast, simple and effective method. Even very small amounts of buffy coat or blood, e.g. 100µl, can be used to isolate MNC.
There is no documented case study in the literature using the density gradient method to separate MNC from such small amounts of blood or buffy coat. Our method is only option for small volumes.
If the user cultivates the MNC in media with fetal calf serum (FCS), it is sufficient to use 2-3% FCS instead of the usual 10% FCS. This helps to reduce the financial burden on laboratories. Please read our publication in the reference section.
Using this protocol, we have developed many cell cultures with MNC over the last 14 years.
Before start
-Please read the protocol carefully.
-Disinfect the working place as well as surfaces of laminar flow.
-Pipette tips must be sterile.
-Laminar flow should be disinfected through UV light for 30 minutes before use.
-Waste collection system must have some disinfectant like Sodium hypochlorite or 70% ethanol etc.
Steps
A. Isolation of MNC from human peripheral blood or buffy coat:
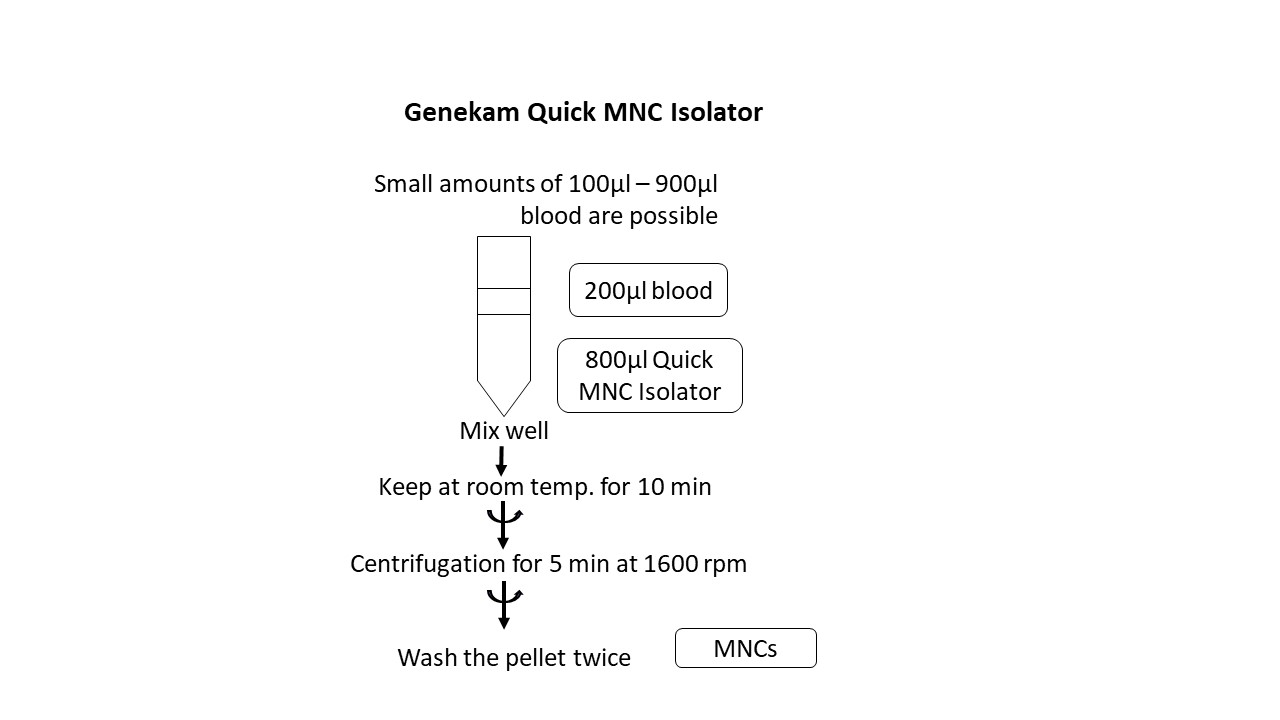
Add 900µl Genekam Quick MNC Isolator to each tube containing up to 100µl whole blood or buffy coat in a sterile tube (1:10). Otherwise, the ratio of solution to sample should be (1:5). For example, if you want to isolate MNC from 2 ml of buffy coat, add 8 ml of Quick MNC Isolator. A sterile 15 ml tube is sufficient for use. Alternatively, a sterile 50 ml tube can be used for larger volumes of blood.
Work with the smallest amount of sample to gain experience with the kit instead of losing the entire sample the first time. Shake each tube gently immediately after adding the solution. Store at room temperature away from light for 10 minutes.
Centrifuge for 5 minutes at 1600rpm. Aspirate or discard the supernatant without disturbing the pellet.
Resuspend the pellet in the appropriate buffer (in most cases this is PBS) and wash it twice in this way.
Centrifuge each time for 5 minutes at 1600rpm and slow down the speed.
If the pellet still contains ethrocytes, treat the sample with Genekam Quick MNC isator and repeat the washing step.
Resuspend the pellet and proceed with further procedures, e.g. counting the MNC, and perform analyses such as cell culture, magnetic separation of specific populations as well as nucleic acid isolation to perform molecular analyses.