Sample collection and eDNA extraction from Sterivex filter units
Oscar E Chiang, Pedro Inostroza
Abstract
The following workflow covers several steps in the DNA analysis of environmental samples, from the water collection to the analysis back in the lab. The samples can be taken from several water systems (i.e. sea, lakes, rivers, streams) and collected in triplicate (1 L) in Sterivex sterile filter units (Merck, cat. no. SVGP01050). The DNA extraction protocol modifies the Dneasy PowerWater Sterivex kit (Qiagen, cat. no. 14600-50-nf).
Steps
Sample collection
A portable peristaltic pump (Vampire sampler; Buerkle) is used for sampling at an approximate 30-50 mL/min flow rate. The tubing inserted in the unit's head has a suction hose (Marprene; 4.8 mm inner diameter), connected to a flexible silicone hose. A suitable adapter connects both hoses.

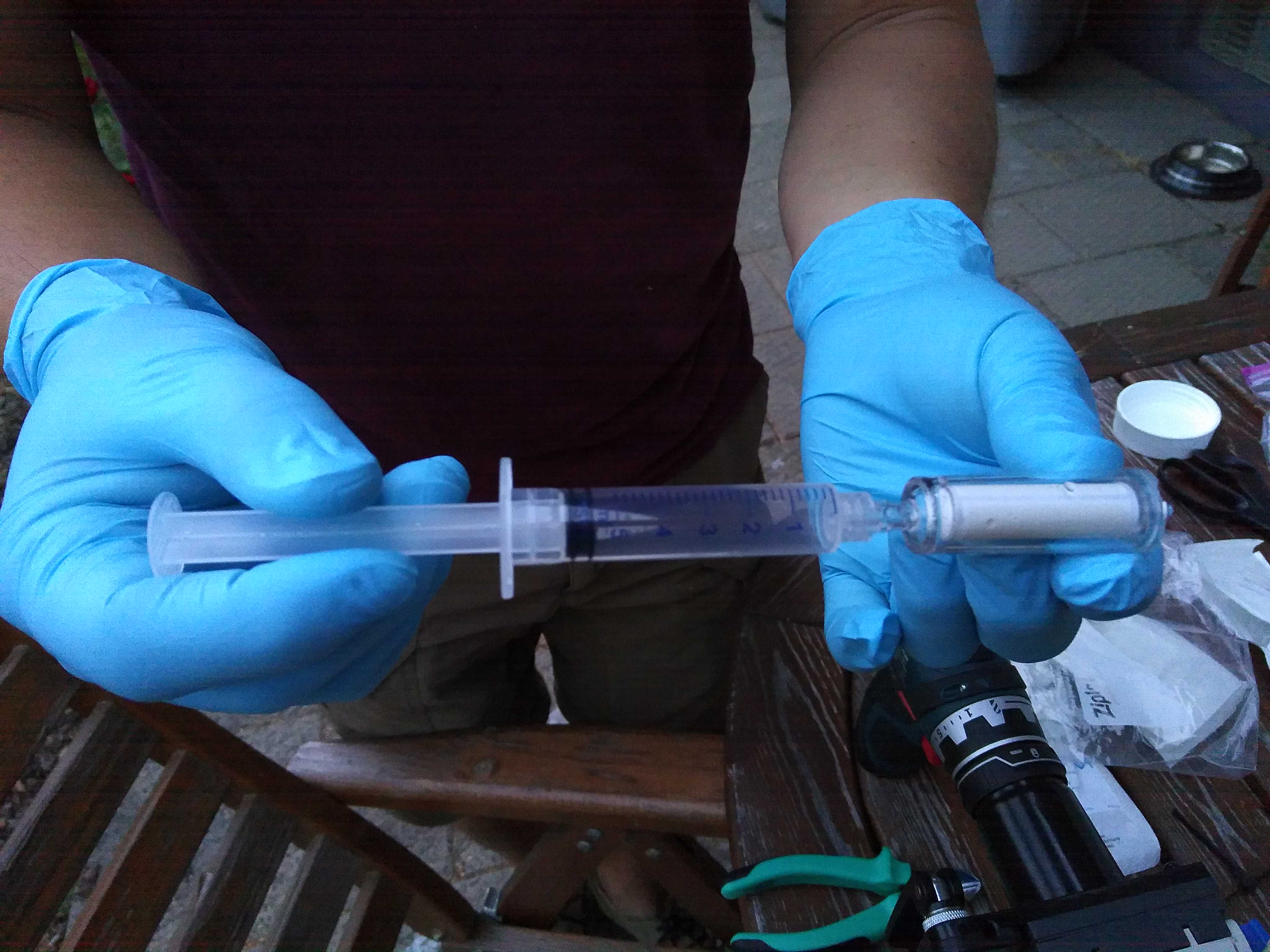
A Sterivex filter unit (0.22 µm pore size) is attached to the suction hose by a stainless steel male Luer-lock ring hose barb (1/4'). We recommend using a hose clip (9-10 mm; Buerkle cat. no. BURK8678-000) to firmly tight the Luer-lock ring to the hose. Each filter can process up to 2L of water depending on the amount of the suspended material.

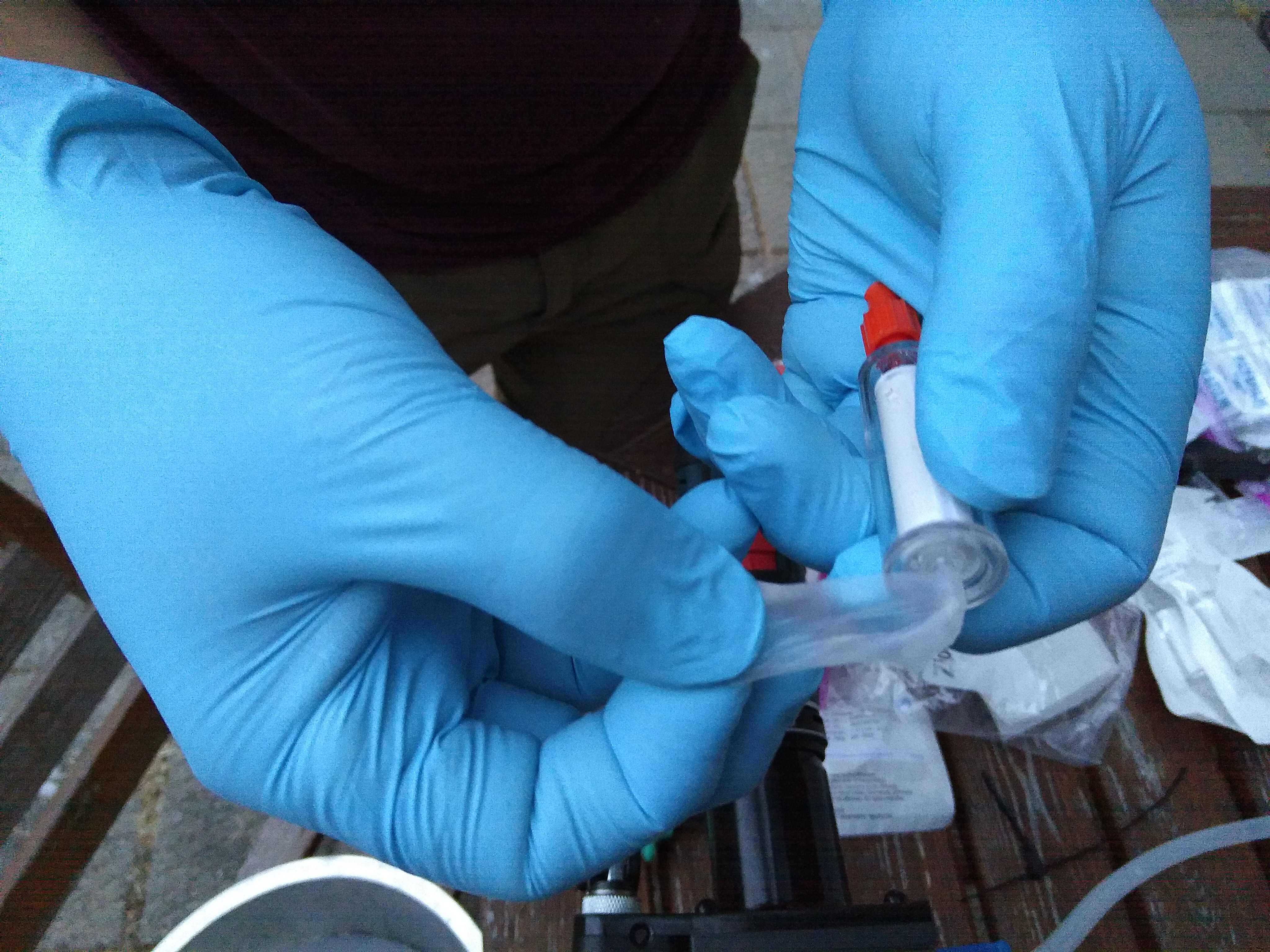
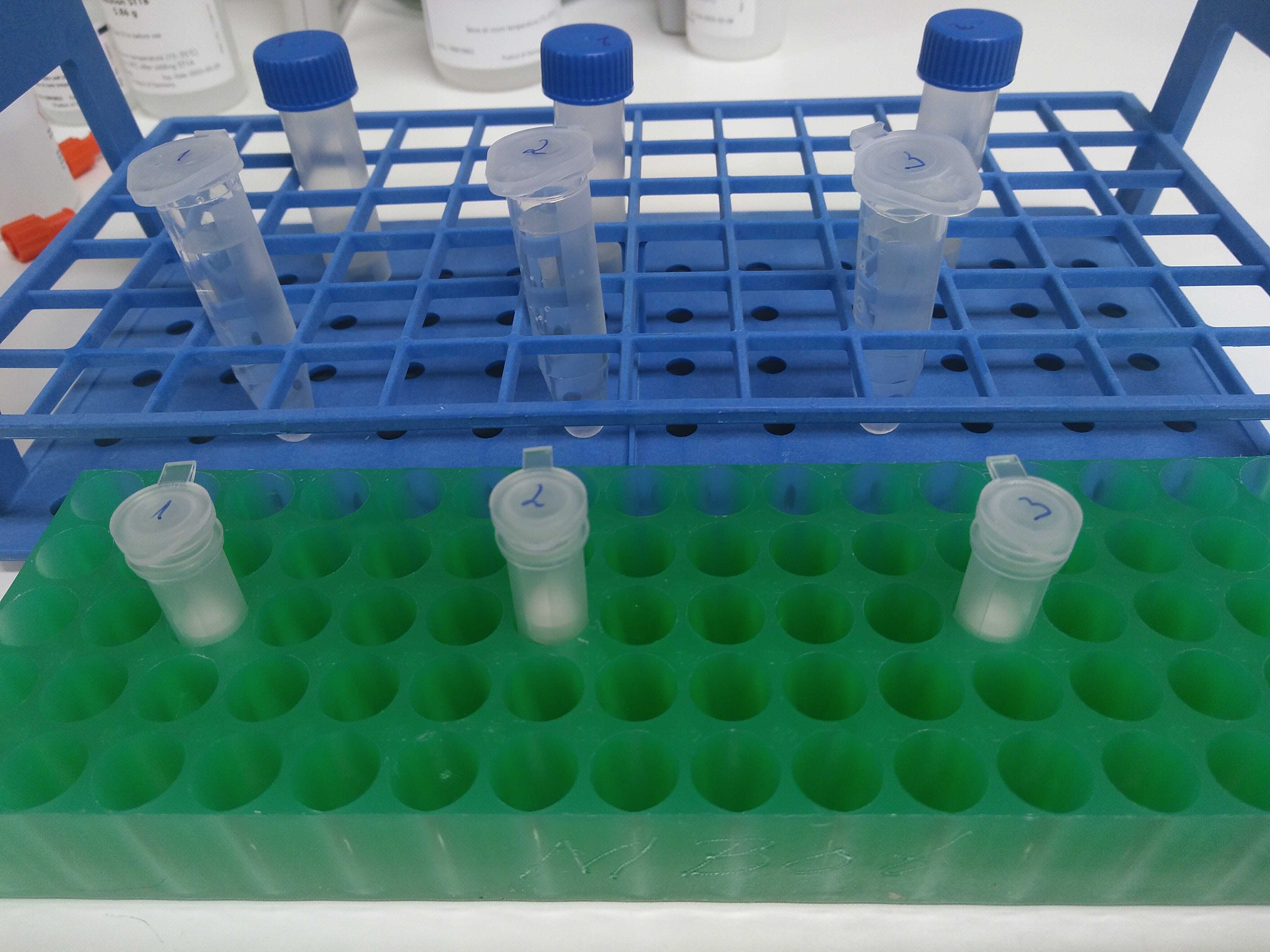
Now that the samples are ready and sealed, do not forget to identify them accordingly. A good rule of thumb is to write down ID, date, replicate number, filtered volume and station on the filter. Do the same on the sample bags, especially if there are more filters per site or replicate. The samples are store at -20°C degrees.

DNA extraction
Allow mixing in a vortex with a horizontal adapter (Qiagen, Cat. No. 13000-V1-5) for 00:05:00 at the minimum speed. Set the filters with the inlet facing out and check for potential leakage from the outlet sealed with Parafilm.

Rotate the Sterivex in 90 degrees and vortex for 0h 5m 0s at the minimum speed. Repeat the previous step 2 more times (4 times x 0h 5m 0s each filter)
Add 900µL of solution MBL. Dispense slowly; a fraction of the volume can be lost.
Before use, heat the MBL solution at 65°C for 0h 10m 0sas is suggested by the manufacturer.
Incubate the filter units vertically with the inlet upward at 90°Cfor 0h 5m 0sin an oven. Before and after the incubation, check for any leakage in the Parafilm; replace it if needed.
Let the filters cool down at room temperature for 0h 2m 0s, and re-tight the caps and check the Parafilm. Then, vortex at maximum speed for 0h 5m 0s. While mixing, check that the filters stay in place. If not, lower the speed.
Centrifuge the tube at 4000x g,0h 0m 0sfor 0h 1m 0s
Carefully transfer the lysate to a clean 2.2mL collection tube. Then, add 1,6µL of RNAse (final concentration 100mg/mL). Incubate the samples at 37°C x 0h 30m 0s in a heating block or a water bath.
Add 300µL IRS solution to the tube and vortex. Then incubate at 4°C for 0h 5m 0s.
Centrifuge the tube at 13000x g,0h 0m 0sfor 0h 1m 0s
A VacValve vacuum system and a vacuum pump are used for the following steps.

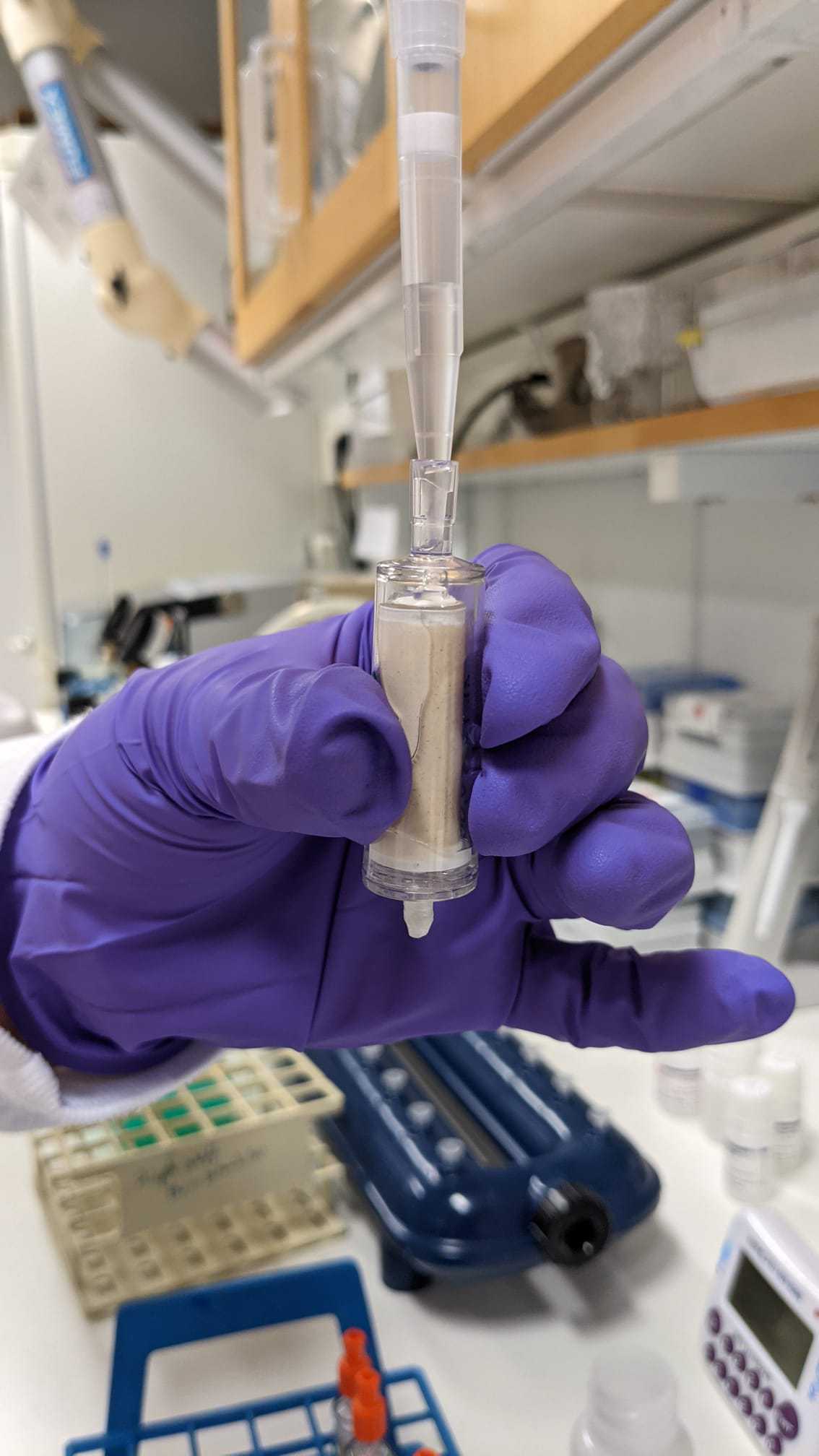
Load the supernatant (4,5mL) into a tube extender and MB spin column. Filtrate at low pressure. The column is attached to the VacValve vacuum system, as shown below.
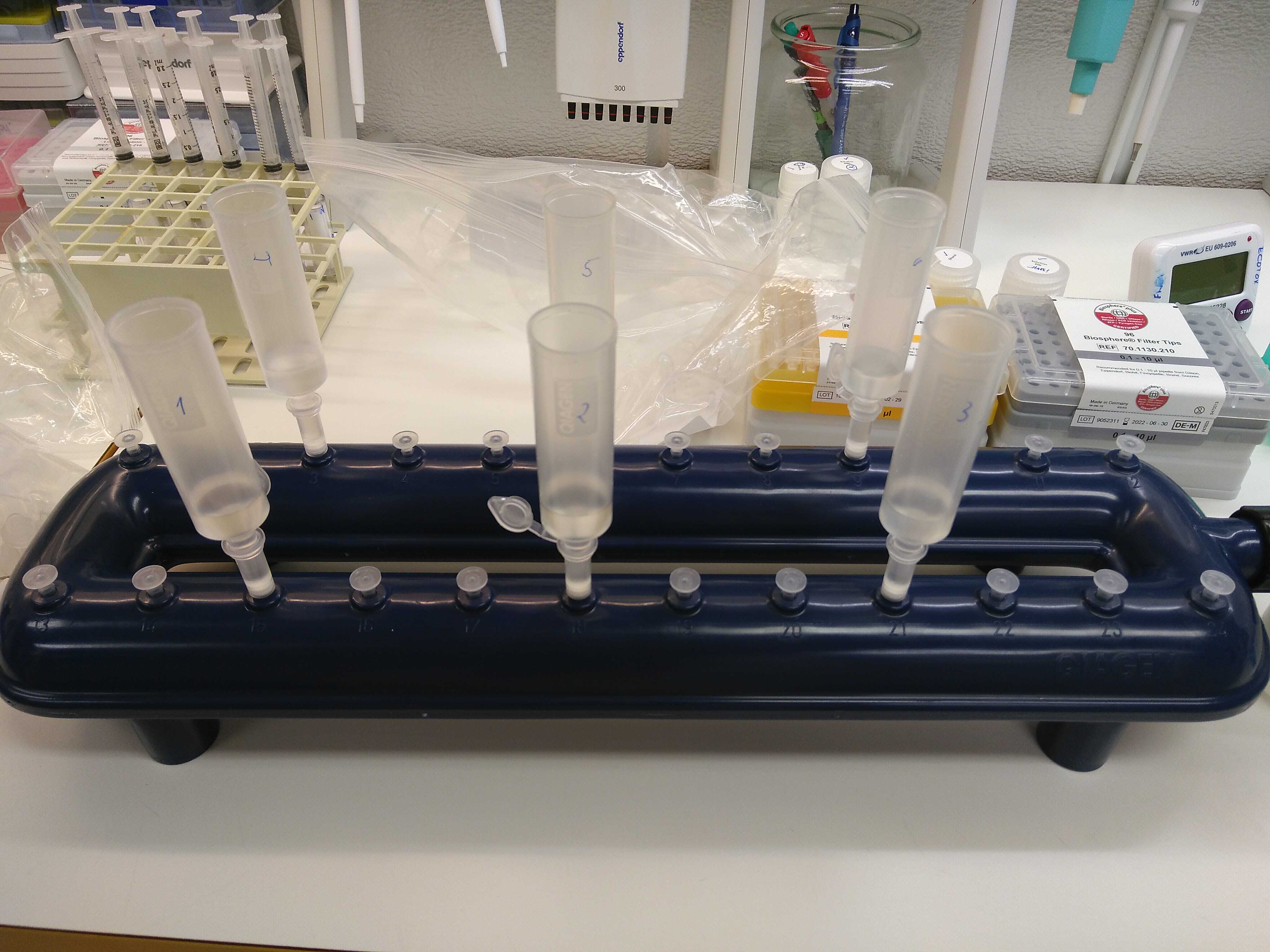
Now, wash the spin column with 800µLof PW solution. Keep the columns open.
Allow the membrane to dry by keeping the vacuum pump running for 1 min.
Repeat step 21, and wash the spin column adding 800µLof ethanol. Allow the membrane to dry as before.
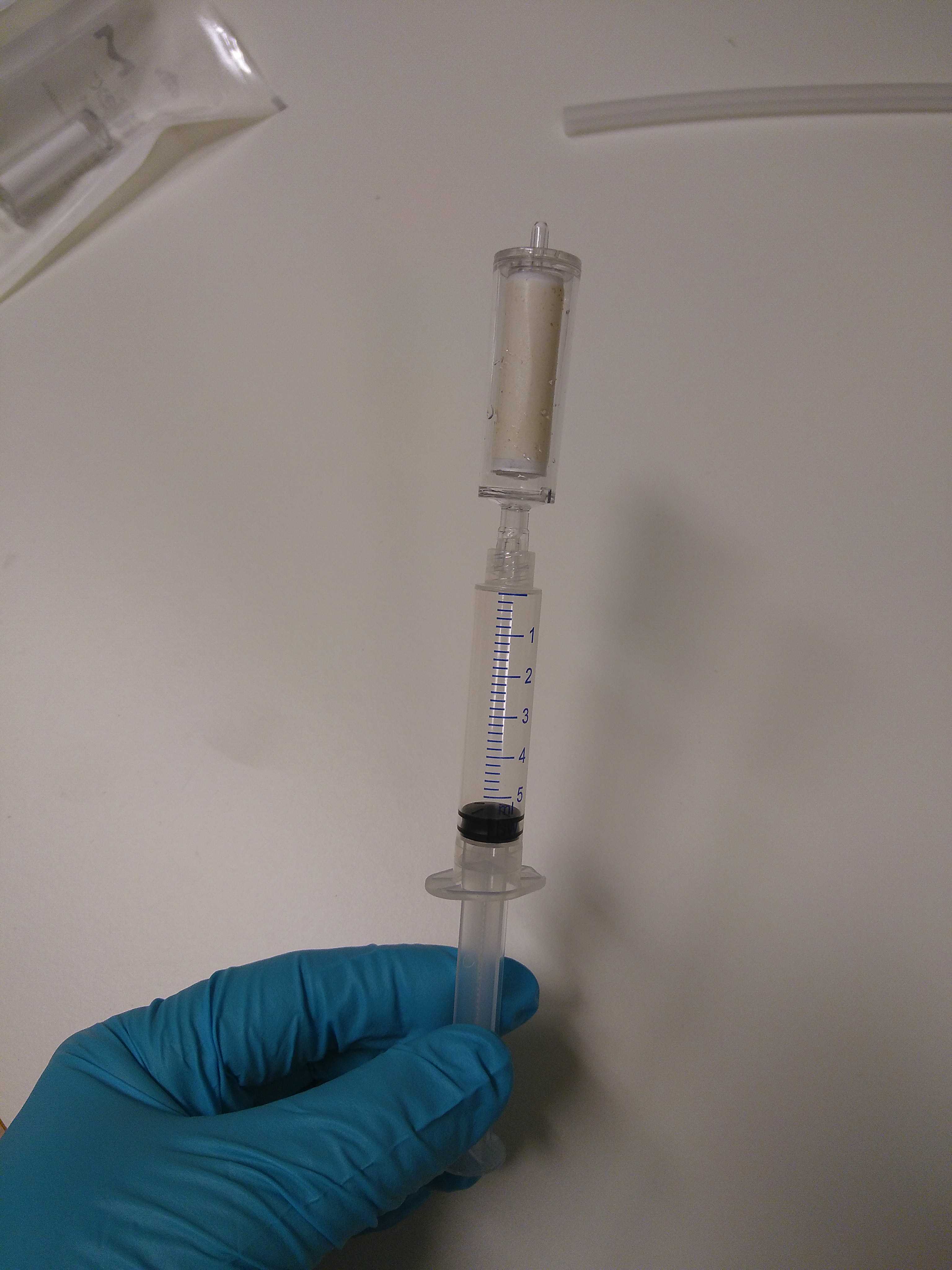
Place the spin column in a clean collection tube and dry it out by centrifugation at 13000x g,0h 0m 0s for
0h 2m 0s
Transfer the spin column to a new clean tube, and add 50-100µLof solution EB (or DNA-free grade
water). Centrifuge at 13000x g,0h 0m 0s for 0h 1m 0s.
Voila! The DNA is in the collection tube, ready for further processing.
DNA quality check
The DNA purity is checked with a Nanodrop.