Retroviral transduction of primary murine CD8 T cells
Tamer B Shabaneh, Andrew R Stevens
Retroviral
Transduction
Primary T cells
CAR
murine
Transfection
Cell culture
dynabeads
retronectin
CD8 T cell isolation
spinoculation
Abstract
This protocol will guide you through the process of how our group (Riddell Lab, Fred Hutchinson Cancer Center) generates murine CD8+ CAR T cells.
We normally begin this protocol on Monday, transduce the cells on Thursday, and immunophenotype them by the following Monday. The CAR T cells can then be used in standard cell-based assays (e.g. cytotoxicity, cell proliferation, or cytokine production) or for in vivo studies. See the following two protocols for details:
_ 51Cr Release Cytotoxicity Assay for murine CAR T cells
_ Standard cell-based assays for cytokine release and cellular proliferation of murine CAR T cells.
The protocol will hopefully elaborate on the usual methods-section write-up that reads something like this:
Murine T cell viral transduction and adoptive transfer: : CD8+ T cells were negatively selected (Stem Cell) from spleen and peripheral lymph nodes of 6–8 week-old CD45.1 mice, and stimulated with 1 mg/ml plate-bound anti-CD3 (145-2C11) and anti-CD28 (37.51) for 18-20 h at 37°C 5% CO2 in complete RPMI (RPMI-1640, 10% heat-inactivated FBS, 1 mM HEPES, 100 U/mL penicillin/streptomycin, 1 mM sodium pyruvate, and 50 μM b-mercaptoethanol) supplemented with 50 U/mL human IL-2 (IL-2+, Peprotech). Retrovirus was captured for 2 h at 2560rcf at 32°C onto wells pre-coated with RetroNectin (Takara) and CD8+ T cells were added at 1×106 cells/mL in IL-2+ complete RPMI and mouse T-activator Dynabeads (ThermoFisher) at 1:1 ratio. Plates were centrifuged at 800rcf for 30 min at 32°C and incubated overnight. T cells were then resuspended in 1.5x volume of IL-2+ media and incubated for an additional 24 h. T cells were resuspended in 3x volume of IL-15+ media and incubated for an additional 48 h. Activator beads were removed and transduction efficiency was determined by flow cytometry. CAR T cells were prepared for infusion by resuspending at the indicated number of CAR T cells per 100 µL serum-free RPMI-1640 and kept on ice prior to adoptive transfer. 6-8 week-old C57BL/6J female mice were pre-conditioned as indicated and 6 h later injected intravenously with 3×106 CAR T cells.
Before start
Prepare reagents listed below.
-
1L cDMEM [1000 mL DMEM (1x), 100mL FBS (heat inactivated), 25 mL HEPES (1M), 10mL L-Glutamine (200 mM), 10 mL Pen/Strep. Filter the mix through 500 mL Bottle top Filter (0.2 μm aPES membrane)].
-
1L mTCM [1000 mL RPMI1640 (w/ 20 mM HEPES) (1x), 100 mL FBS (heat inactivated), 10 mL Sodium Pyruvate (100 mM), 1 mL HEPES (1 M), 10 mL Pen/Strep, 100 uL 2-Mercaptoethanol (0.5 M). Filter the mix through 500 mL Bottle top Filter (0.2um aPES membrane)].
-
500 mL EasySep Buffer [500 mL D-PBS (1x), 10 mL FBS (heat inactivated), 1 mL EDTA (0.5 M). Filter the mix through 500mL Bottle top Filter (0.2 μm aPES membrane)].
-
500 mL 2% BSA blocking solution [500 mL D-PBS (1x), 10 g dry weight Bovine Serum Albumin. Filter through 500 mL Bottle top Filter (0.2 μm aPES membrane)]. Combine and let dissolve for >15 minutes prior to filtering.
-
500 mL Flow Buffer [500 mL D-PBS (1x), 2.5 mL FBS (heat inactivated), 3.6 mL EDTA (0.5 M)]. No need to filter.
-
Diluted Retronectin (12.5 μg/mL diluted in 1x D-PBS and aliquotes stored at -20C until use).
-
1000x human IL-2 stock (50,000 IU/mL in 1x D-PBS; reconstituted per manufacturer). hIL-2 is cross-reactive and is more economical.
-
1000x human IL-15 stock (10,000 IU/mL in 1x D-PBS; reconstituted per manufacturer). hIL-15 is cross-reactive and is more economical.
Attachments
Steps
Day 1: Plating the plat-E cells
Approximately 20 hours prior to transfection of platinum-E (plat-E) cells, generate a working stock of 0.001% (v/v) poly-L-lysine (PLL) by diluting 0.1% PLL stock 1:100 in 1x D-PBS and coat a 6-well-plate well with 1.5 mL for 0h 15m 0s.
Aspirate PLL and replace with 2 mL pre-warmed cDMEM.
Dissociate plat-E cells using Trypsin-EDTA (0.05%), resuspend the cells at 7.5×105 / mL in cDMEM, and transfer 1 mL to the PLL-coated well (final volume = 3 mL). Rock the cells to ensure even monolayer formation and incubate at 37 °C, 5% CO2, 95% humidity overnight.
Day 2: Transfection of plat-E cells for retroviral packaging
Transfect Plat-E cells for retroviral packaging using
Starting in the afternoon: In a sterile 1.7 mL microcentrifuge tube, dilute 7 μg of plasmid in Xfect buffer to a final volume of 100 μL. Vortex briefly and pulse centrifuge the tube.
Add 2.25 μL Xfect Polymer to each tube. Immediately vortex for at least 10 seconds at high speed.
Incubate 0h 10m 0s
Vortex again briefly and pulse centrifuge.
Apply Xfect mixture dropwise to the respective well and gently rock the plate to mix.
Place transfection plate in the incubator (37 °C, 5% CO2, 95% humidity) overnight.
Following the overnight incubation, exchange the transfection medium (on day 3) with fresh pre-warmed cDMEM and incubate the transfected plat-E culture for additional 24-30 hours. Harvest the supernatant (on day 4) and filter through 0.45 μm. Collected retroviral supernatant can be snap-frozen and placed at -80 °C long-term. Otherwise, the retroviral sup is ready for immediate use to transduce murine T cells.
Prepare the plate-bound antibodies [on day 2] for T cell stimulation for the following day [day 3]:
Dilute anti-CD3 (1 mg/mL) and anti-CD28 (1 mg/mL) antibodies 1:1000 in PBS. Coat the required number of wells in a 6-well non-TC plate with 1 μg/mL anti-CD3 and 1 μg/mL anti-CD28 in PBS (4 mL/well). Incubate 4°C.
Day 3: Isolation of murine lymphocytes and their activation
Prepare the following for the trip to the vivarium:
-
Ice bucket
-
6-well plate with 4 mL mTCM per well (1 well per mouse)
-
Sterile, curved medium-point forceps
-
Sterile, stainless steel surgical scissors
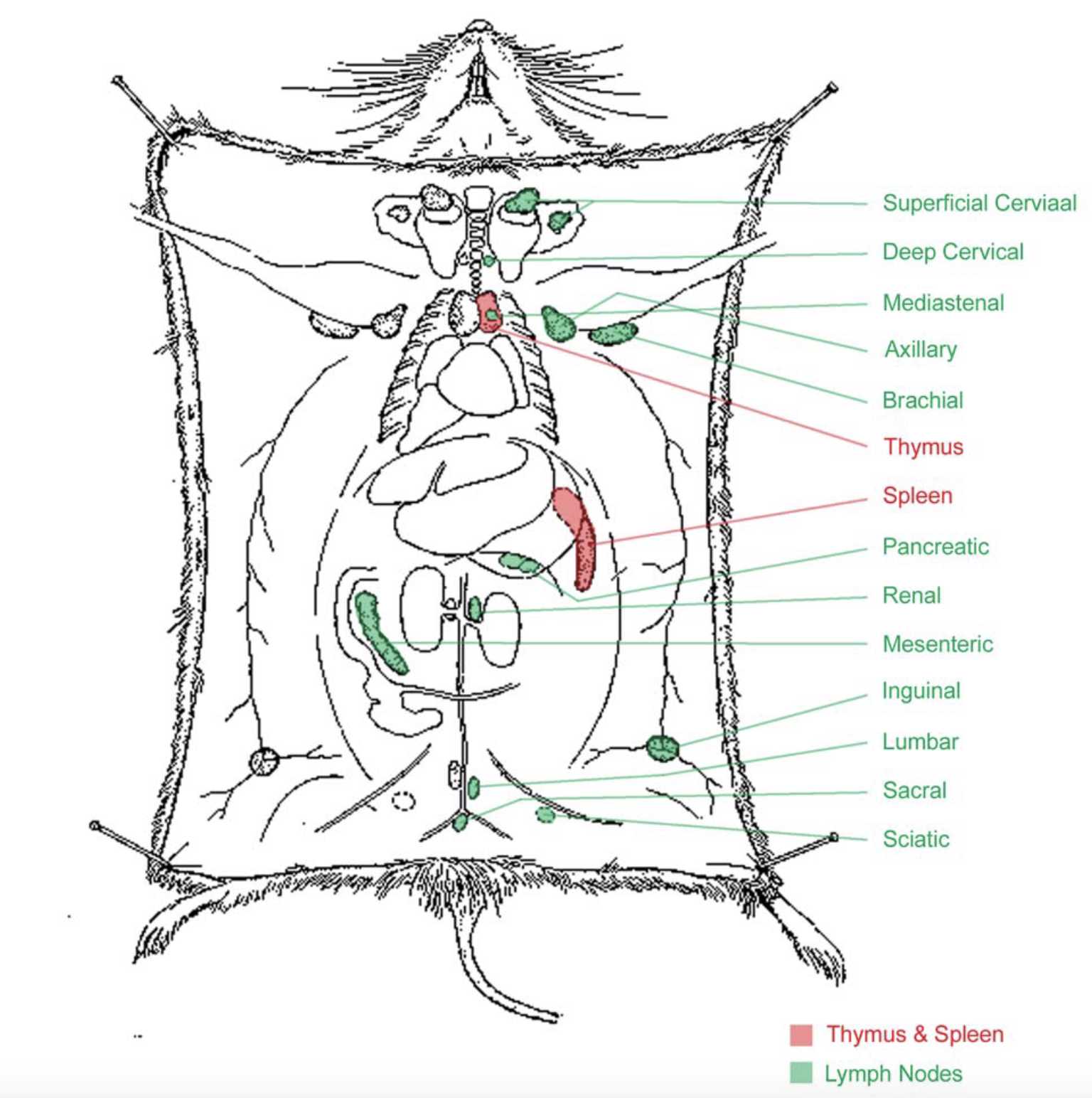
Euthanize mice per IACUC-approved protocol, and harvest the spleen and lymph nodes (inguinal LNs can be sufficient, but adding brachial, axillary, and superficial cervical LNs will increase the CD8 T cell yield).
Process harvested LN and spleen in a biological safety cabinet.
Mechanically dissociate spleen and LNs by repeatedly pressing and grinding them between the frosted surfaces of two glass slides. Collect the dissociated tissue in a new 6-well-plate well with fresh mTCM.
Filter the cell suspension into a 15 mL conical tube through a 100 μm cell strainer. Rinse each well with with 1 mL mTCM and filter through the same strainer.
Isolate CD8 T cells using the
Centrifuge the filtered cells (from step 8) 400rcf. Remove supernatant with a pipet, being careful to avoid the cell pellet. Re-suspend in 1 ml StemCell buffer per mouse-equivalent in tube. Add 20 μL FcR blocker (equivalent to 50 μL Normal Rat Serum) and 50 μL CD8-isolation antibody cocktail each per mouse-equivalent and incubate0h 10m 0s.
Add 125 μL RapidSpheres per mouse equivalent and incubate 0h 5m 0s.
Add StemCell buffer up to a volume of approximately 8-9 mL, place uncapped 15 mL conical in "The Big Easy" magnet for 0h 5m 0s.
Carefully pour the enriched T cell suspension into a new 15 mL conical. Centrifuge cells to pellet 400rcf. Re-suspend in 10 mL mTCM. Enumerate T cells using a hemocytometer or automated cell counter.
Prepare cell culture media by diluting recombinant human IL-2 (IL-2) in mTCM to a concentration of 50 IU/mL (mTCM+IL-2)
Centrifuge cells to pellet 400rcf. Re-suspend cells to a concentration to 1x106 cells/mL in mTCM + 50 IU/ml IL-2.
Remove the PBS solution from CD3/28-coated plate (prepared on day 2 and stored at 4 °C) and add up to 5 mL T cell suspension to each coated well. Incubate at 37°C
Prepare recombinant human fibronectin (RetroNectin)-coated plate in preparation for following day (day 4):
Coat a non-TC-treated 24-well flat bottom plate with 400 μL 12.5 μg/ml RetroNectin in 1x D-PBS per well. Prepare 1 well per 1x106 T cells to be transduced, with the addition of 1 well for a non-transduced negative control.
Incubate 4°C.
Day 4: Transduction of T cells
Transfer the RetroNectin solution (incubated from day 3) to a second non-TC-treated 24-well flat bottom plate.
Set aside and incubate 4°C.
To the first RetroNectin-coated plate, add 500 μL 2% BSA in 1x D-PBS per well. Block the wells by incubating 0h 30m 0s.
While the wells are being blocked, return to the transfected plat-E cultures to prepare the retroviral sup for T cell transduction. Following the 24-30 hour incubation (from day 3), collect retroviral sup from the Xfected Plat-E cells. Filter through 0.45 μm filter.
Rinse the blocked RetroNectin wells with 1 mL 1x D-PBS, aspirate, then add 1 mL fresh retrovirus sup to each blocked well.
Carefully wrap each covered 24-well plate in tin foil to minimize evaporation, balance, and centrifuge
2560rcf,32°C
Before the retroviral capture is completed, collect the T cells activated on day 3. Suspend and enumerate cells by hemocytometer or automated cell counter. Centrifuge to pellet 400rcf. After centrifugation, re-suspend at a concentration of 1x106/mL in fresh mTCM+IL-2.
Rinse enough Gibco Dynabead mouse T-activator CD3/28 (Dynabeads) to achieve a final 1:1 ratio with T cells: aliquot Dynabeads to 9 mL fresh mTCM and place in the EastEight EasySep magnet. Incubate 0h 5m 0s. Aspirate mTCM being careful to avoid Dynabeads. Combine the T cells with Dynabeads at 1:1 ratio and briefly set aside.
Collect viral capture plate from centrifuge.
Work quickly - one well at a time - such that wells do not dry out. one well at a time - such that wells do not dry out.
Aspirate virus supernatant and rinse the well with 1 mL 1x D-PBS. Aspirate PBS using the pipette, then transfer 1 mL homogenous cell suspension onto the virus-captured well.
Once T cells are added, re-wrap the virus-coated plate in foil to minimize evaporation. Centrifuge 800rcf,32°C.
When complete, unwrap foil, place the cells in the incubator 37°C.
Day 5: Culturing T cells in IL-2 mTCM
Collect T cells from incubator. Mix the T cells well with a P-1000 pipette and transfer into sterile micro-centrifuge tubes. Centrifuge samples 400rcf. Aspirate supernatant while being careful to avoid the cell pellet. Re-suspend each cell pellet in 1.5x the initial volume, 1.5 mL mTCM+IL-2.
Retrieve the second RetroNectin plate that was placed into 4 °C (on day 4). Aspirate the old Retronectin and transfer T cells into respective wells. Incubate 37°C.
Day 6: Culturing T cells in IL-15 mTCM
Prepare cell culture media by diluting recombinant murine IL-15 (IL-15) in mTCM to a concentration of 10 IU/mL (mTCM+IL-15)
Mix T cells well with a P-1000 piepette and transfer to a 15 mL tube. Rinse plate-well with 1 mL mTCM and transfer to respective tube. Enumerate T cells by hemocytometer or automated cell counter. Centrifuge 400rcf. Re-suspend to adjust concentration to 1x106 cells/mL mTCM+IL-15.
Incubate 37°C
Day 8: Immunophenotyping to assess the transduction efficiency
Transfer each cell culture to 15 mL conical tubes. Remove Dynabeads by placing the tubes onto EasyEight EasySep Magnet for 2 minutes. Transfer the "de-beaded" culture by pipetting into new 15 mL conical tube. Enumerate by hemocytometer or automated cell counter.
Immunophenotype the samples with the appropriate markers (e.g. CD8, transduction marker, congenic marker, etc..) by flow cytometry.
If desired, further enrich transduced cells by sorting or using magnetic isolation kits targeting a transduction marker.
Prepare cells for in vivo experiment and/or setup in vitro assays. See the following two protocols for details:
_ 51Cr Release Cytotoxicity Assay for murine CAR T cells
_ Standard cell-based assays for cytokine release and cellular proliferation of murine CAR T cells.
If not needed immediately, CAR T cells can be cultured for an additional day before use. To culture for an additional day, centrifuge cells 400rcf, then re-suspend them at a concentration of 1x106/mL mTCM+IL-15. Place back in incubator 37°C.

