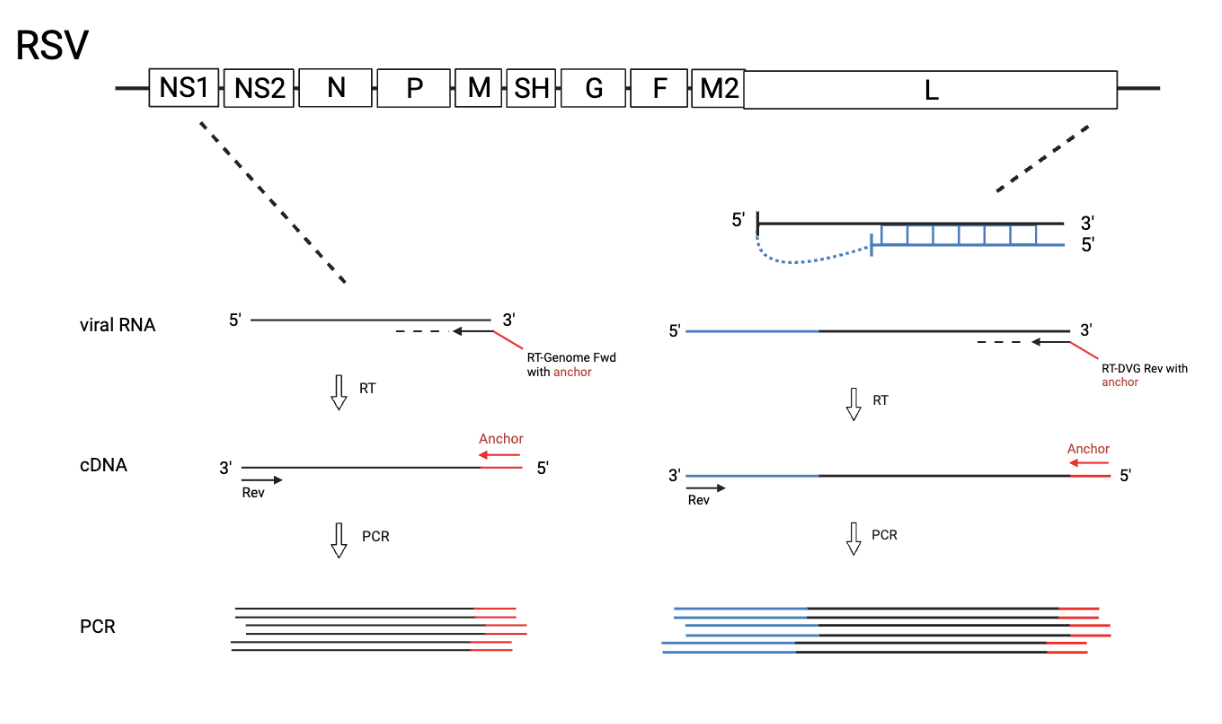
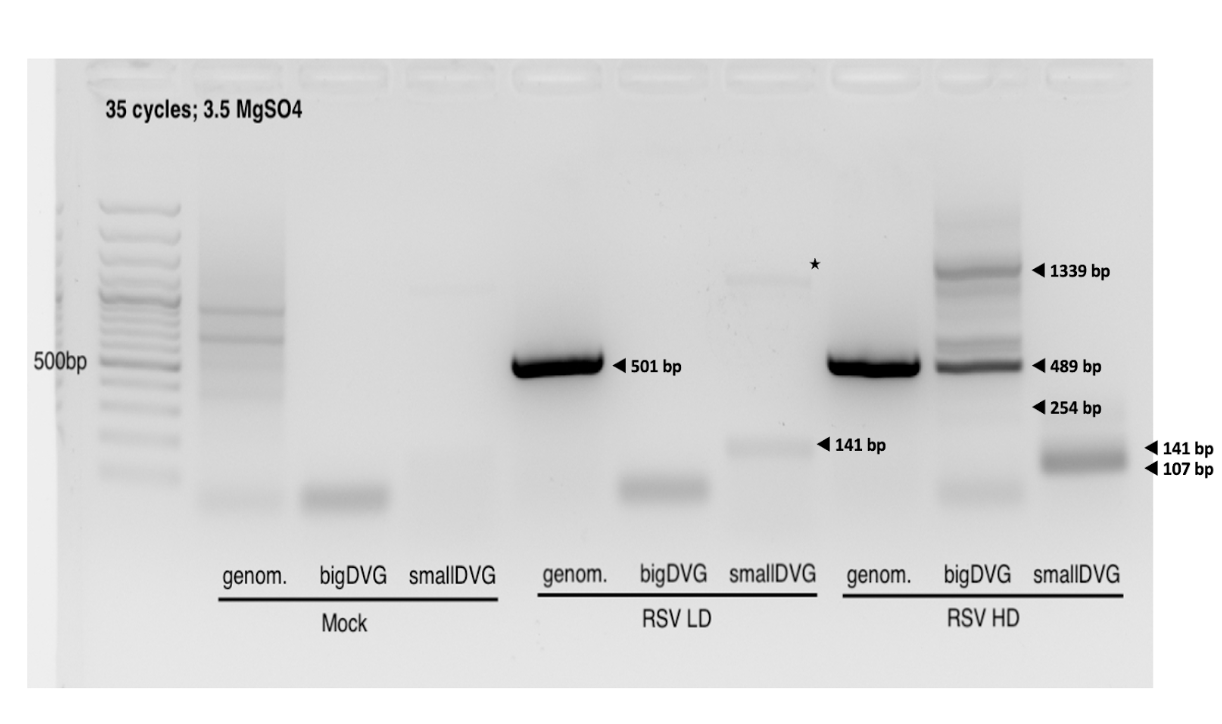
RSV standard and copyback genomes PCR Protocols
Carolina Lopez
Abstract
Protocol for the detection of the Respiratory Syncytial Virus (RSV) standard and copyback genomes by PCR
Before start
PRINCIPLES BEHIND THE PROCEDURE MUST BE UNDERSTOOD. PLEASE CONSULT WITH EXPERIENCED LAB MEMBER THE FIRST TIME YOU USE THIS PROCEDURE. UPDATE AS A GENERAL PROCEDURE AS NECESSARY BUT DO NOT MODIFY WITH SPECIFICS TO YOUR PROJECT, INSTEAD DOWNLOAD AND PASTE A MODIFIED COPY IN YOUR NOTEBOOK.
Steps
RSV standard and copyback genomes PCR Protocols
*The “Big” DVG design can detect cbVGs with break before 14882 and rejoin before 15112 (RSVA2). The “Small” DVG design can detect cbVGs with break before 15112 and rejoin before 15194 (RSVA2). The “Small” DVG design should theoretically also pick up big DVGs, but bigger amplicons do not get amplified as well. “gRSV rev” has been tested as an RT primer for both “Big” and “Small” DVG design, however, gave an unspecific amplicon with the “Big” DVG design at around 500bp. As a consequence, two separate RT and PCR reactions must be run when wanting to look for both big DVGs and small DVGs . For most experiments, it is enough to only use the “Big” DVG design. RSV Genome can also be detected using this protocol when using the Genome primers. Primer locations are 387-410 and 894-918 (RSVA2), however not an optimal match (seems to have been designed for RSV B?).
Procedure
Reverse Transcription (RT): *RT reaction can be completed in a 10 μL reaction or a 20 μL reaction. The 10 μL reaction is preferred, if RNA concentrations allow, to help conserve reagents.
-
Start with
500ngof RNA diluted in dH2O (range 200-2000ng (background increases with more RNA))`10µL` reaction: dilute in `4µL` dH<sub>2</sub>O `20µL` reaction: dilute in `8µL` dH<sub>2</sub>O -
Add primer (
2micromolar (µM))`10µL` reaction: `0.5µL` `20µL` reaction: `1µL` -
Add dNTPs
`10µL` reaction: `0.5µL` `20µL` reaction: `1µL` -
Incubate at
65°Cfor0h 10m 0sProgram in PCR Machine: RT-DI1 -
Prepare the mix
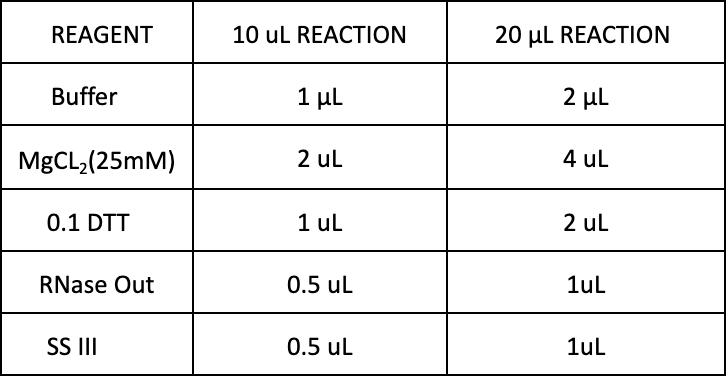
-
Add mix to sample
`10µL` reaction: `5µL` `20µL`reaction: `10µL` -
Incubate at
50°Cfor0h 50m 0sthen85°Cfor0h 5m 0sProgram in PCR Machine: RT-DI2 -
After program is finished keep at
-20°Cfor at least0h 20m 0s -
Spin down tubes in mini microcentrifuge
-
Add RNase H (add in the PCR hood! DVGs are very stable!)
`10µL` reaction: `0.5µL` `20µL` reaction: `1µL` -
Incubate at
37°Cfor0h 20m 0sProgram in PCR Machine: RNaseH -
Keep in
-20°Cfor at least0h 20m 0sbefore moving on to the PCR
PCR
- Prepare master mix (can be done on bench)
*Volumes below are for when 1 μL of cDNA is used. Adjust dH2O volume accordingly if more cDNA is added. Up to 4 μL cDNA can be added but increases background. Total reaction volume should equal 25 μL.
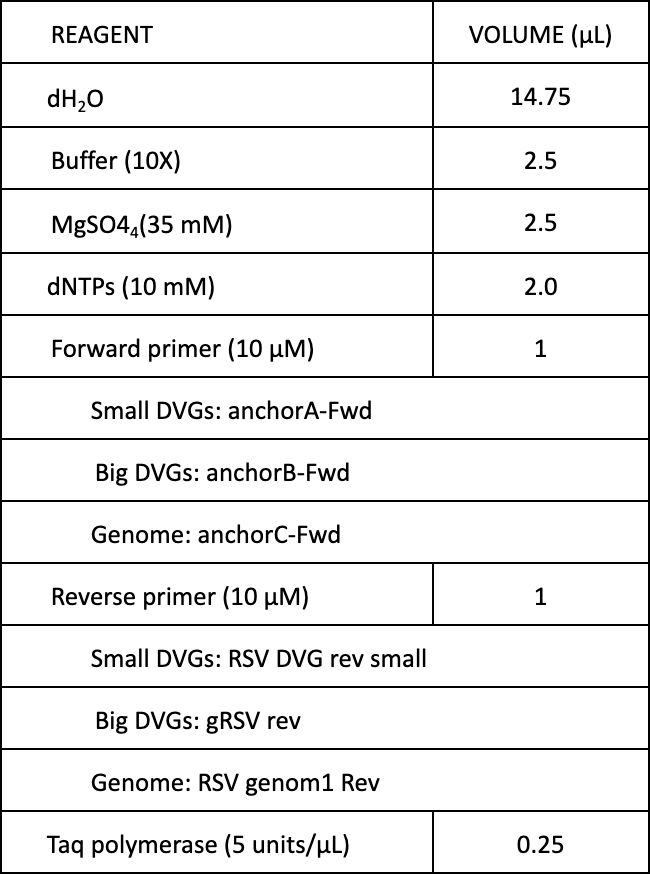
-
Add
24µLof master mix to PCR tubes (can be done on bench) -
Thaw cDNA samples and spin down in mini microcentrifuge
-
Add
1µLof cDNA sample to master mix (add in PCR hood! DVGs are very stable!) -
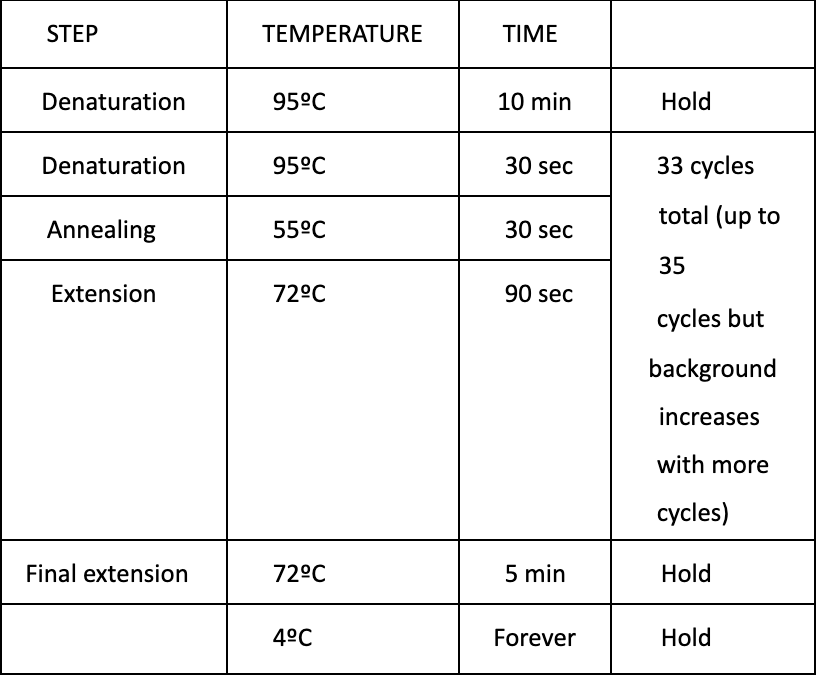
Run PCR
Program in PCR Macine: RSVDI233
Gel Electrophoresis:
-
Prepare 1% agarose gel
1 g pure agarose in `100mL` of 1X TAE buffer Microwave until agarose solution dissolves completely (`0h 2m 0s`) Let agarose solution cool before adding Ethidium bromide (if you can keep your fingers on the flask without burning, then it is at an appropriate temperature) Add `1-5µL` of Ethidium bromide and mix by swirling flask -
Pour agarose solution into gel cast (remember to put in the well comb)
-
Let the agarose solidify (wait at least
0h 30m 0s) -
Place gel in electrophoresis chamber containing 1X TAE buffer (make sure buffer covers the gel)
-
Load ladder and samples to wells
`6µL` of Ladder Ladder stock recipe: `100µL` GeneRuler 100 bp Plus DNA ladder from Thermo Scientific: SM0321 `100µL` DNA Gel Loading Dye (6X) from Thermo Scientific: R0611 `400µL` dH<sub>2</sub>O `30µL` of Sample + Loading dye mix (dilutes from 6X to 1X) `5µL` of DNA Gel Loading Dye (6X) from Thermo Scientific: R0611 `25µL` of sample -
Run at 110 volts for
0h 30m 0s -
Analyze gel bands