RNA Extraction and RT-qPCR of Human Lung Organoids
Morris Baumgardt, Stefan Hippenstiel, Andreas C. Hocke, Katja Hönzke, Maren Hülsemann, Maren Mieth, Doris Frey
Disclaimer
Informed written consent was obtained from all volunteers and the study was approved by the Charité Ethics Committee (project 451, EA2/079/13).
Abstract
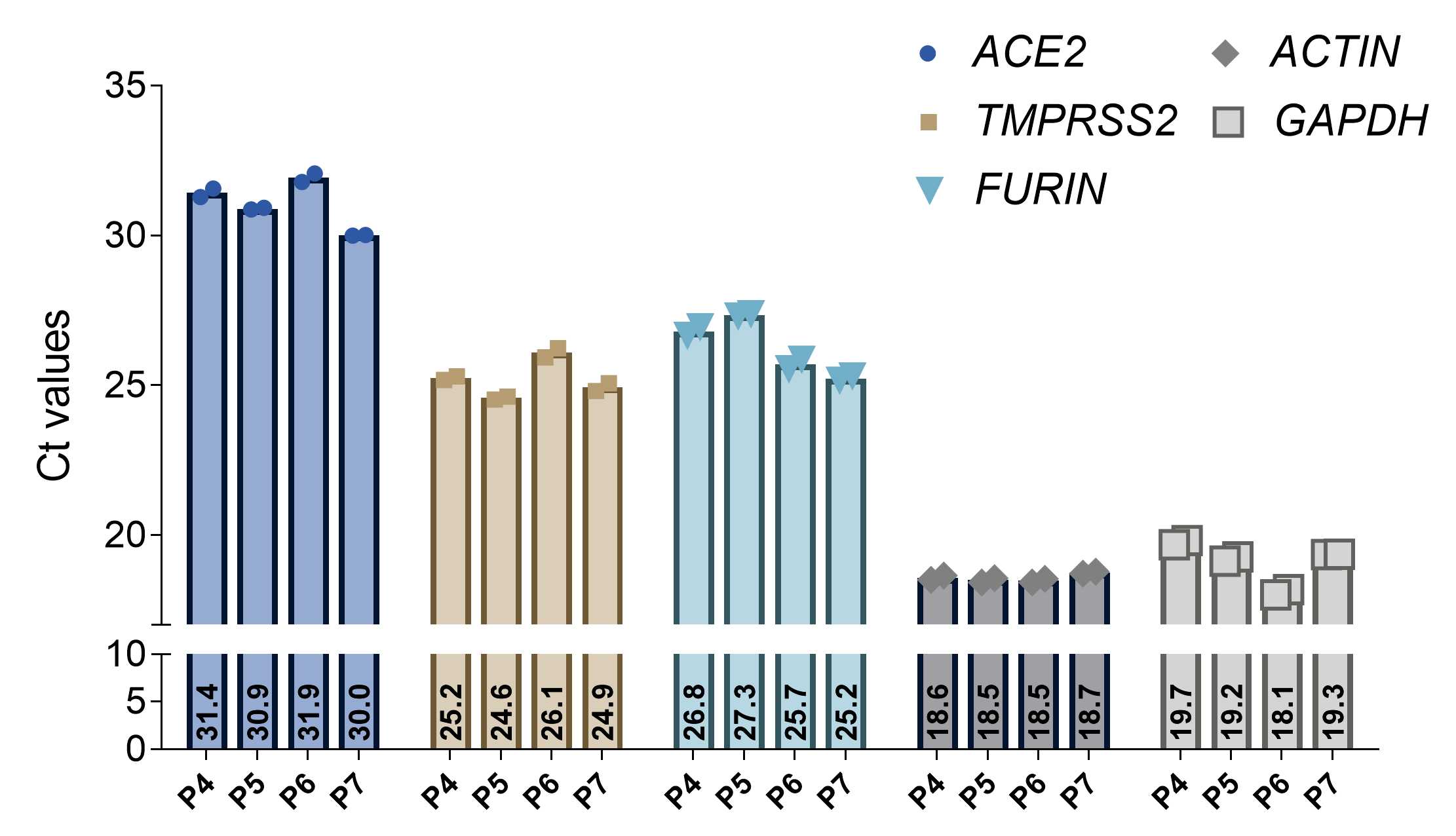
This protocol describes the RNA extraction from human alveolar-like organoids followed by the perfomance of a RT-qPCR to quantify the amount of hostfactors ( ACE2 , TMPRSS2 , and FURIN ) of SARS-CoV-2. The protocol contains detailed steps for the lysis of human alveolar-like organoids, as wells as the RNA extraction followed by the reverse transcription into cDNA. In the next section the protocol describes in detail how to quantify the gene expression with the TaqMan gene expression kit to obtain donor dependent Ctvalues.
Before start
To avoid contaminations RNAase/DNAase free reagents, consumables and filter tips must be used at all steps.
Please use RNA low bind tubes.
Steps
Organoid lysis for RNA isolation
Grow your 3D model as described.
Remove the entire organoid medium from the wells.
Add 1mL ice-cold base medium and collect Cultrex with organoids in a 2 mL tube and flush well with additional 1mL base medium and collect also.
To disolve the Cultrex place the tube at 4°C for 0h 5m 0s.
Centrifuge at 900x g,4°C.
Carefully remove supernatant and add 350µL RLT Puffer + β-Mercaptoethanol to the organoids (pellet).
Place supernatant in a 1.5 mL safelock tube.
Isolate RNA using „RNeasy Mini Kit” from Qiagen (Cat No./ID 74104).
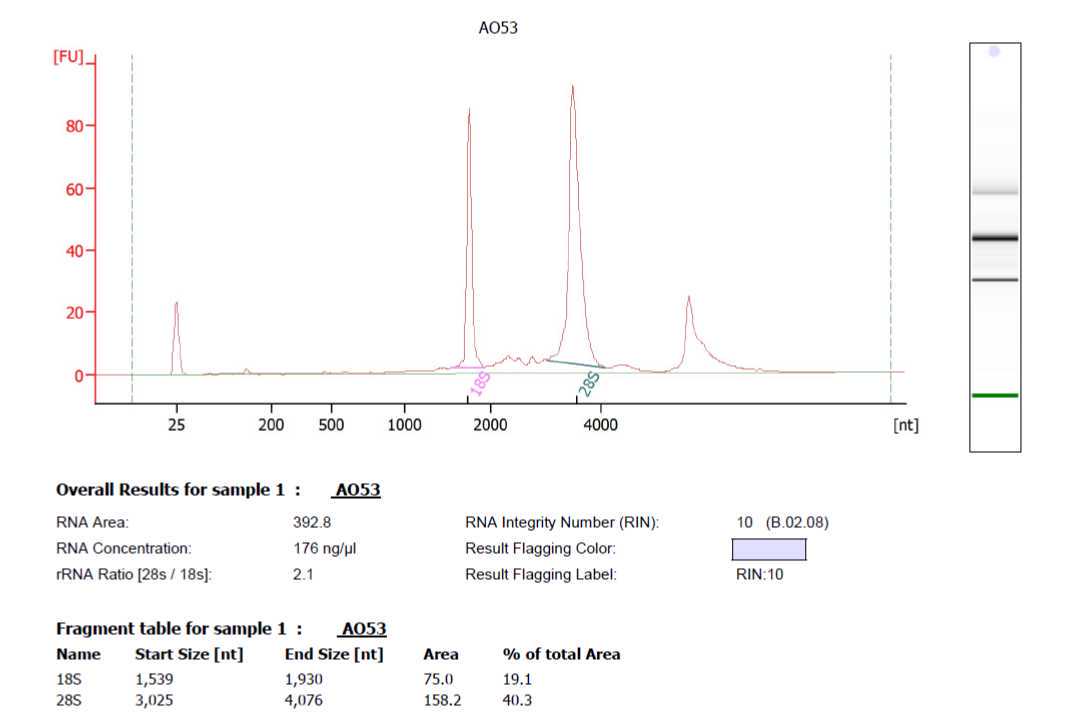
After isolation, measure RNA quality via RIN (RNA Integrity Number) using 2100 Bioanalyzer instrument (manufacturer's instructions, no changes have been made to the protocol included in the kit "Agilent RNA 6000 Nano Kit"). High-quality RNA will contain an RIN of at least 8. All samples used in this protocol had a RIN of 10.
Preparation of 2x RT master mix and cDNA reverse transcription
All steps 4On ice.
To quantify the amount of RNA before reverse transcription use 1.5µL of your RNA solution using the NanoDrop.
Allow the kit components to thaw on ice (high capacity cDNA reverse transcription kit, Applied biosciences).
Calculate the volume of components needed to prepare the required number of reactions.
Prepare the RT master mix on ice.
One reaction needs:
| A | B |
|---|---|
| Reagent | Amount (µL) |
| 10x RT Buffer | 2 |
| 25x dNTP Mix (100mM) | 0.8 |
| 10x RT Random Primers | 2 |
| MultiScribe™ RT | 1 |
| Nuclease-free H2O | 4.2 |
| TOTAL | 10 |
Master mix (reverse transcription)
According to NanoDrop-measurement prepare an RNA/water solution containing 1µg RNA/10µL water in a thin wall PCR tube. If RNA concentration is low, 500ng RNA/10µL water is also sufficient.
Include RT control: use water instead of RNA, run one control with each primer.
Add 10µL of 2x reverse transcription master mix to each tube.
Use the following program for reverse transcription:
| A | B | C |
|---|---|---|
| Step | Temperature | Duration |
| 1 | 25°C | 10 min |
| 2 | 37°C | 2 h |
| 3 | 85°C | 5 min |
| 4 | 4°C | infinite |
Program for Reverse Rranscription in a Thermal Cycler
Add 80µL H2O to each tube (cDNA).
Use the cDNA directly for qPCR or store at -20°C.
TaqMan - quantitative PCR
Prepare the qPCR master mix. Calculate the volume of components needed to prepare the required number of reactions for each Gene expression assay.
| A | B |
|---|---|
| Reagent | Amount (µL) |
| Gene Expression Master Mix | 10 |
| H2O | 4 |
| TaQMan Gene Expression Assay | 1 |
| Total | 15 |
TaqMan Gene Expression Assay Master Mix (1 Reaction)
Perform qPCR, pipetting 15µL of respective TaqMan gene expression assay master mix to the bottom of the well, then pipet 5µL of cDNA on the upper wall of the well.
Seal the plate with a Clear Adhesive Film.
Centrifuge the plate for 1200x g.
Important quality controls:
Run each sample in duplicates.
Always run house keeping genes (e.g., GAPDH and ß-ACTIN ) and gene of interest on the same plate.
Include water control for each primer.
Include RT control from previous step.
Run the qPCR reaction in a Thermal Cycler:
| A | B | C | D |
|---|---|---|---|
| Stage | Temperature | Duration | Repetitions |
| 1 | 50°C | 2 min | 1 |
| 2 | 95°C | 10 min | 1 |
| 3 | 95°C | 15 s | 40 |
| 4 | 60°C | 1 min | 40 |
qPCR Program (PCR Volume = 20 µL)