Purification of synaptic vesicles from rodent brains, troubleshooting and column cleaning/regenerating
Amberley Stephens
Synaptic vesicle
vesicle purification
rat
size exclusion chromatography
gel filtration
column pouring
Abstract
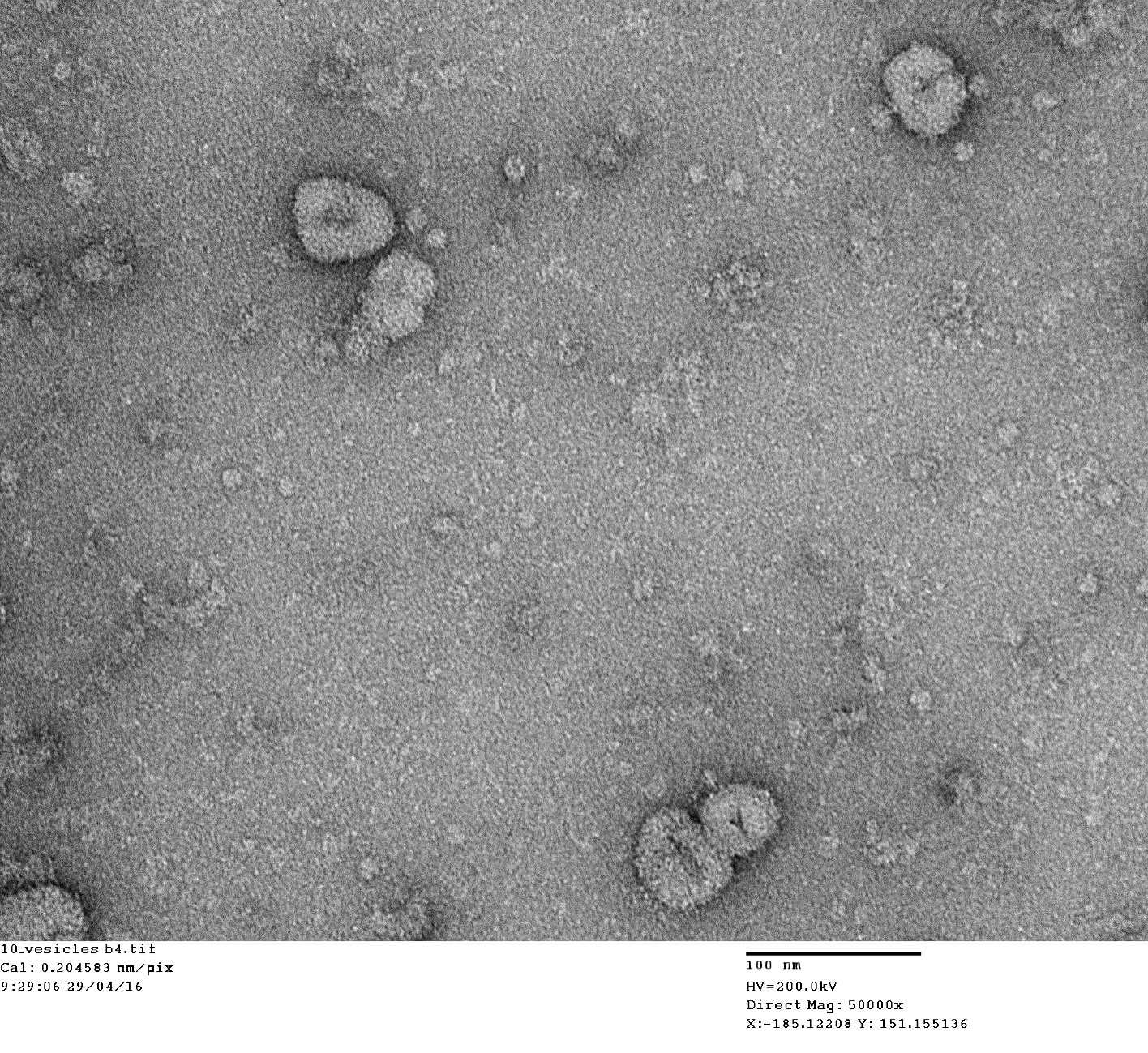
Purification of synaptic vesicles from tissue samples is initially performed using sucrose gradients and ultracentrifugation. To obtain highly pure synaptic vesicle samples an additional size exclusion chromatography step is required, but can be tricky to optimise and troubleshoot. This protocol presents additional information for pouring of the chromatography column, saturating the column, cleaning the column and troubleshooting for lack of and/or loss of resolution. The purification protocol followed is from Ahmed S, Holt M, Riedel D, Jahn R. Small-scale isolation of synaptic vesicles from mammalian brain. Nat Protoc. 2013 May;8(5):998-1009
Steps
We follow the protocol provided by Ahmed, et al 'Small-scale isolation of synaptic vesicles from mammalian brain (2013)', which has proved to be a reproducible and well-designed protocol. We will only mention adaptations we made as we follow most of the protocol step by step.
We use the brains of two Sprague Dawley rats per preparation. We homogenise each brain in 9 mL of homogenization buffer and pooled the two preps at Part 1, Step 9.
For all of Part 1 and Part 2, Steps 6 and 7 we use
Equipment
| Value | Label |
|---|---|
| Sorvall LYNX 4000 | NAME |
| Superspeed Centrifuge | TYPE |
| Thermo Scientific™ | BRAND |
| 13493336 | SKU |
| https://www.thermofisher.com/us/en/home.html | LINK |
Equipment
| Value | Label |
|---|---|
| A27-8 x 50 | NAME |
| Fixed Angle Rotor | TYPE |
| Thermo Scientific™ | BRAND |
| 75003008 | SKU |
| https://www.thermofisher.com/us/en/home.html | LINK |
For Part 2 Steps 10 onwards we use
Equipment
| Value | Label |
|---|---|
| Optima XL-90 | NAME |
| Ultracentrifuge | TYPE |
| Beckman | BRAND |
| No longer sold | SKU |
| https://www.beckmancoulter.com | LINK |
For the sucrose gradients we make the CS1 supernatant to a volume of 31 mL with homogenising buffer and layer 6 x 5 mL gradients. We use the SW41 rotor at a speed of 30,000 rpm (the ultracentrifuge can only be input in rpm). This equates to ~111,000 x g RCF av, ~154,000 x g RCF max.
Equipment
| Value | Label |
|---|---|
| 13.2 mL 14 x 89mm | NAME |
| Open-Top Thinwall Ultra-Clear Tube | TYPE |
| Beckman Coulter | BRAND |
| 344059 | SKU |
| https://www.beckmancoulter.com | LINK |
Equipment
| Value | Label |
|---|---|
| SW 41 Ti | NAME |
| Swinging-Bucket Rotor | TYPE |
| Beckman Coulter | BRAND |
| 333790 | SKU |
| https://www.beckmancoulter.com | LINK |
Part 3. purity check by dot blotting.
This step doesn't need to be repeated once you are happy with the reproducibility of the first two parts of the protocol.
We used their suggested antibodies against Rp4
Size Exclusion Chromatography - setting up the column
Part 4: size-exclusion chromatography as a final purification step.
This is the most complicated and time consuming part of the process.
The column needs to be filled at least 90% full of Sephacryl otherwise you will not get the separation between peak 1 and peak 2 containing the vesicles.
In the protocol they say you require 70 mL of packed resin, but i found i needed more.
I found the easiest way to pour the column was to use an orbital shaker and a pump (see figure)
The orbital shaker keeps the media in suspension so it is added to the column in a more homogeneous suspension - note the pumping tube needs to be stuck to the media bottle while shaking!
The pump was set to 1 mL/min which seemed a good speed to add the chromatography media. As the media is in suspended in liquid, every now and again I let it settle and opened the valve at the bottom of the column to drain the liquid to allow more chromatography media to be added.

Size Exclusion Chromatography - saturating the column
You will not observe vesicles coming out of the column in the first few runs, it seems the column needs to be saturated first. I emailed the authors who suggested i could try BSA if i wanted, but i felt happier just using the brain samples. It took 4 rounds of the protocol to get a protein signal, so 8 brains in total.
Size Exclusion Chromatography - peak resolution
I measure the protein concentration of each fraction by hand using a nanodrop as we don't have an absorbance measurement system attached to the fraction collector. The point of sample elution changes during each run. I see two peaks, as shown in the paper. If only one peak is present either more sephacryl is needed in the column, or a smaller volume of sample should be added to the column, I try to add 200-400 uL.
To clean the sephacryl, if resolution is reducing or there is contamination. I invert the column and pump the sephacryl out into a beaker. I allow it to settle and remove the liquid. I put it on an orbital shaker and add 0.5 M NaOH and allow it to mix for a few hours before letting the sephacryl settle and pouring off the NaOH. I then rinse with water buy putting it back on the orbital shaker, then letting it settle and pouring off a couple of times. I then reload the sephacryl back into the column in 20% Ethanol.