Protein Extraction and Western Blot of Human Lung Organoids
Morris Baumgardt, Stefan Hippenstiel, Andreas C. Hocke, Katja Hönzke, Maren Hülsemann, Diana Fatykhova
Disclaimer
Informed written consent was obtained from all volunteers and the study was approved by the Charité Ethics Committee (project 451, EA2/079/13).
Abstract
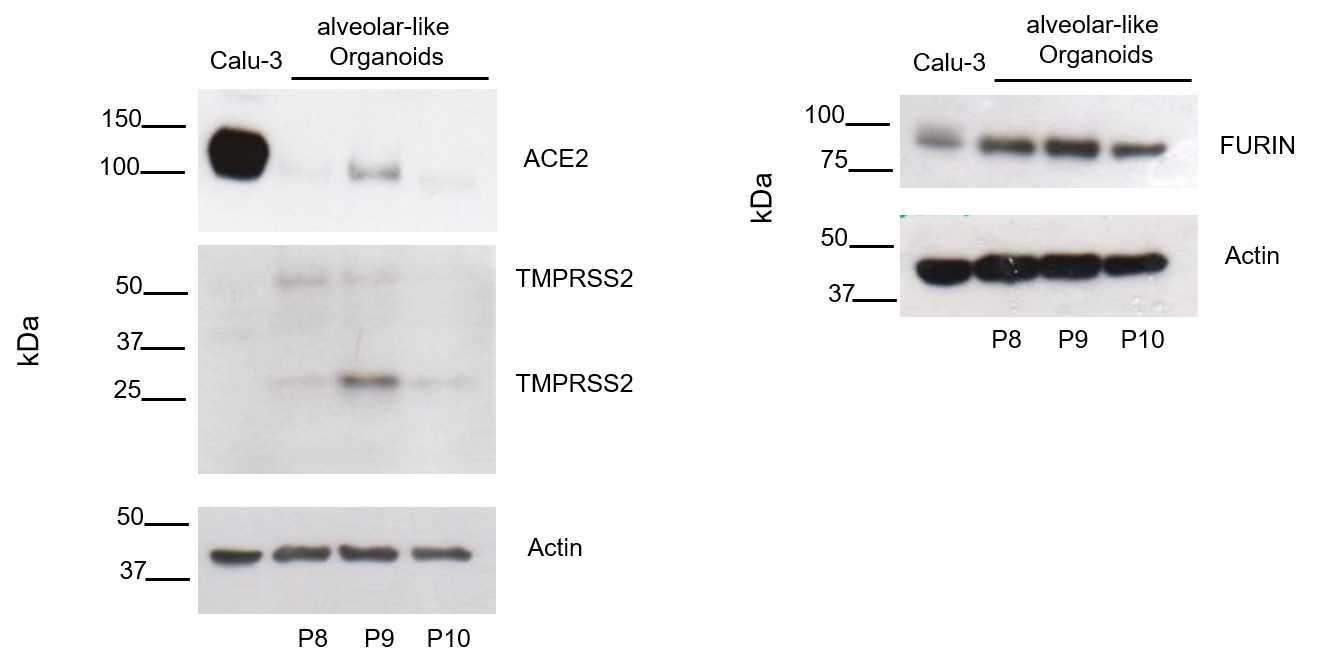
This protocol describes the protein extraction from human alveolar-like organoids followed by western blotting. It gives a detailed description of the preparation of cell lysate from human alveolar-like organoids, followed by exact steps to perform a western blot and the immunostaining to detect ACE2, TMPRSS2, and FURIN as host factors of SARS-CoV-2.
Before start
Please use protein low-bind tubes for all steps.
Steps
Prepare cell lysate of alveolar-like organoids
You need a sufficient amount of organoids (approx. 300,000 cells per well) and organoids should be collected from minimum two wells of a 24-well plate (total ~600,000 cells).
From here all steps 4On ice.
Keep tubes, reagents and buffers on ice.
Remove organoid media by aspiration.
Add 1mL ice-cold PBS to the organoids.
On ice Transfer organoids to 15mL LoBind Tube (with 1000 µL pipette).
Centrifuge 300x g,4°C.
On ice Remove supernatant carefully.
4On ice Make sure the protease inhibitor is added to the RIPA buffer.
Add 75µL RIPA buffer + Protease inhibitor to pellet.
Breake organoid pellet by repeated resuspension (3 times) using a disposable syringe with 27G needle.
Transfer sample to 1.5 mL LoBind tube.
Centrifuge 16000x g,4°C.
On ice Collect and transfer supernatant to 1.5 mL LoBind tube (~50µL).
Freeze at -80°C or continue.
Protein quantification and preparation before Western blot
Use 5µL of RIPA-lysate in DC Assay (a dilution of the sample might be required, 1:10 or 1:50 in PBS, follow manufacturer's instructions) for protein quantification.
Prepare appropriate amount of sample by using 50µg protein lysate according to DC assay results with 4x Laemmli protein sample buffer for SDS-PAGE.
Shake for 0h 7m 0s at 95°C (1000rpm,0h 0m 0s).
SDS-PAGE
Commercial gel used: Bio Rad 7.5% (Mini-Protean TGX Precast Gels Cat# 456-1023).
Arrange gel chambers inside the electrophorator and fill the tank with 1x Running-Buffer (fill the chamber formed by the gels with new 1x Running-Buffer, check if everything is tight and fill the tank with 1x Running-Buffer until it is half full). Remove the comb carefully.
Load 5µL Kaleidoscope Protein Standard in lane 1 (Precision Plus Protein Kaleidoscope Prestained Protein Standards #1610375).
Add 50µg sample/lane (can be increased to 100 µg sample/lane for low expressed proteins).
Run the gel for up to 1h 30m 0s at 100 V (until the blue front line runs out of the gel).
Blotting
After the SDS-Page is done, do not switch off the power (due to diffusing proteins) until everything is prepared for blotting. It is better to reduce the voltage instead.
Before use keep bottle of 1x blotting buffer in the freezer for 1h 30m 0s. Equilibrate PVDF membrane (0.45micromolar (µM) methanol for 0h 0m 15s) and transfer it to the 1x blotting buffer. Add sponges and filter papers in 1x blotting buffer. For each gel, you need 2 sponges and 4 filter papers.
Carefully open gel chamber and equilibrate gel in 1x blotting buffer for 0h 2m 0s.
Create a transfer sandwich as follows:
- White side of retainer (top)
- Sponge
- 2 filter papers
- PVDF Membrane
- SDS Gel
- 2 filter papers
- Sponge
- Black side of retainer (bottom)
Check out that there are no air bubbles between the gel and the membrane. Use a pipette to squeeze out extra liquid and bubbles.
Relocate the sandwich to the transfer apparatus. Add ice and fill with 1x Blotting-Buffer until the sandwich is completely covered.
Transfer for 1h 0m 0s at 100 V.
Blocking and antibody incubation
Wash membrane with PBS.
Block membrane in Odyssey blocking solution for 1h 0m 0s.
Cut membrane for multiple protein detection.
Add primary antibody (diluted in Odyssey blocking solution pure) and incubate membrane on a shaker overnight at 4°C.
Wash membrane thrice with 1x PBS-T for 0h 5m 0s each.
Incubate membrane for 1h 0m 0s at room temperature with secondary antibody solution (in 1x PBS-T with 5% milk).
Wash membrane twice with 1x PBS-T for 0h 5m 0seach.
Wash membrane with 1x PBS for 0h 5m 0s.
Prepare ECL solution and incubate on membrane according to manufactures instructions (0h 2m 0s for Thermo Scientific Kit and 0h 5m 0s for Amersham Kit). The more sensitive Amersham Kit is used for ACE2 detection, all other proteins are detected using the Thermo Scientific Kit.