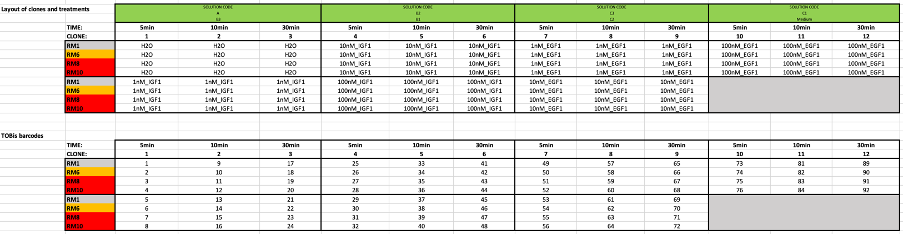
Processing of fixed spheroids for TOBis barcoding, enzyme-free dissociation and antibody staining for CyTOF
Ralitsa R Madsen
Abstract
This protocol is an adaptation and extension of the original work on single-cell signalling in organoids by the Tape Lab at UCL. In this adaptation, I have optimised a new approach for non-enzymatic single-cell dissociation of fixed, scaffold-free spheroids. The adapted protocol is also shorter and uses less material throughout.
Its development would not have been possible without the initial help provided by the Tape Lab in 2021-2022, particularly by Jahangir Sufi. For their original protocol, please refer to the following publication: Sufi, J., Qin, X., Rodriguez, F.C. et al. Multiplexed single-cell analysis of organoid signaling networks. Nat Protoc 16 , 4897–4918 (2021). https://doi.org/10.1038/s41596-021-00603-4
Steps
Spheroid collection
Generate spheroids in Elplasia plates (96-well or 24-well plate format) according to the following protocol: dx.doi.org/10.17504/protocols.io.3byl4bnrrvo5/v1
At the end of your experiment, add 16 % formaldehyde straight to the culture medium for a final dilution to 4 % (e.g., for 96-well plate - add 50µL 16 % formaldehyde to 150µL medium solution; for 24-well plate - add 500µL 16 % formaldehyde to 1.5mL medium).
NB: I recommend using an electronic multichannel pipette at low dispensing speed.
Leave the plate on ice and covered with foil for 1h 0m 0s
Using non-filter aspiration tips, remove the fix solution and add 200µL PBS to each well in a 96-well Elplasia plate or 2mL to wells in a 24-well plate.
NB: if working with 24-well Elplasia plates, to avoid aspirating the spheroids, I recommend using non-filter, gel-loading tips.
It is possible to parafilm the plate at this point and store in the fridge for further processing. I have tested this for plates stored up to 4 months with successful results. Longer storage periods may also work but have not been tested by me or members of my lab.
TOBis barcoding
Depending on the number of samples to be multiplexed, thaw either the 35-plex or 126-plex barcodes at room temperature just before use. Mix and short-centrifuge to pull contents to the bottom of the tubes.
Remove the old PBS from the wells, and replenish with 72µL (96-well) or 270µL (24-well) MaxPar PBS.
Parafilm the plate and leave on a rocker 1h 0m 0s at 4°C
Excess barcode neutralisation with glutathione (GSH)
Once the incubation period is complete and just before you need it, make up ‘x’ ml of 2 mM Glutathione (GSH) in MaxPar CSB (Cell Staining Buffer), for example: 49.2 mg of GSH to 80 ml of CSB, dissolve at room temperature by shaking for 10-20 minutes.
NB: adjust the amount depending on the total number of plates being processed and total volume that will be needed
Remove the barcode solution from each well, then wash with 200µL (for 96-well plate) or 1mL (for 24-well plate) of 2 mM GSH in CSB. Incubate for 10 minutes, then repeat the wash/incubation another 2 times for a total of 3 times.
NB: continue to use gel tips for aspiration of solutions in 24-well plate.
Next, wash with 200µL (for 96-well plate) or 1mL (for 24-well plate) of PBS. Incubate for 10 minutes, then repeat the wash/incubation once more for a total of 2 times.
NB: continue to use gel tips for aspiration of solutions in 24-well plate.
TissueGrinder single-cell dissociation of barcoded spheroids
Pre-coat a TissueGrinder tube with 10mL CSB, making sure to invert the tube to cover all parts, including the blades. Reuse the coating solution to coat an additional 50 ml Falcon for spheroid collection.
NB: for up to 126 conditions with unique barcoding, a single Tissue Grinder tube is needed for dissociation. Increase as needed for multiple independent experiments.
Using the final PBS on the spheroids and CSB-precoated tip (P200 for 96-well; P1000 for 24-well), pipette up-and-down forcefully and transfer the spheroid suspension to a collection boat, then to the CSB pre-coated 50 ml Falcon using a CSB pre-coated 10 ml stripette . Add 5mL PBS to the boat, and use to perform one final wash of each well (multichannel), pipetting across all wells in turn and collecting into the final suspension.
Spin the spheroids down at 800x g , (this is very important, otherwise the spheroids will dislodge when the centrifuge stops).
NB: if the spheroids have not pelleted properly, remove as much of the supernatant as possible without touching the spheroids, then use CSB pre-coated tips to transfer the spheroids to CSB pre-coated 1.5 ml Eppendorf tubes (use several if necessary) and repeat the centrifugation using a benchtop centrifuge at 800x g
Alternative to spin: you can also let the spheroids settle under gravity. Leave unperturbed for 5 minutes.
Remove as much of the supernatant as possible, then use a CSB pre-coated tip to resuspend the spheroids in MaxPar PBS supplemented with 2 mM EDTA for a final volume of approximately 800µL (taking into account the volume taken up by the spheroids).
Keeping the TissueGrinder tube lid inverted on a flat surface and using a CSB pre-coated tip , transfer the spheroid suspension into the lid, fitting it between the blades, then screw on the tube in inverted orientation.
Proceed with dissociation using the standard TissueGrinder protocol "Harsh" (0h 3m 0s).
Spin down the TissueGrinder tube with single cells at 800x g .
Without removing the supernatant, wash the blades/strainer with another 2mL CSB to retrieve any cells that may have been left behind. Repeat centrifugation at 800x g
Gently, remove the supernatant and use a CSB pre-coated tip to resuspend the pellet in 1mL CSB, followed by transfer of the suspension to a CSB pre-coated 1.5 ml Eppendorf tube.
Count the cells using a haemocytometer (I have found that this is more reliable for fixed, dissociated cells compared to automated counters; if the suspension is dense, dilute a small aliquot up to 1:10 in PBS for counting).
Calculate how many cells will be needed for staining (up to ~4 million cells can be used per staining round).
NB: the dissociated cells can be stored in CSB for up to 4 weeks at 4°C.
Extracellular antibody staining
Prepare the extracellular antibody staining cocktail. This requires individual antibody optimisation and titration. An example is provided below, with emphasis on the use of non-overlapping metals (also, checks against intracellular targets). The final volume should be 50µL, with CSB as the diluent.
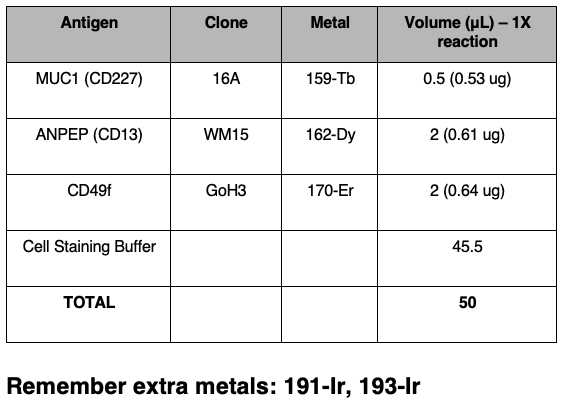
Using a CSB pre-coated tip, take forward the required amount of dissociated cells for staining in 1.5 ml Eppendorf tube and centrifuge in a benchtop centrifuge at800x g
Discard the supernatant and proceed with addition of the 50µL extracellular antibody cocktail.
Incubate for 0h 30m 0s with continuous vortexing at low speed (use tape to secure the tube in an upright position on the vortex).
Once the previous step has completed, add 1mL CSB to the suspension and centrifuge in a benchtop centrifuge at1500x g
Discard the supernatant.
Permeabilisation
Resuspend the cell pellet in 200µL of 0.1 % Triton X-100 (diluted in MaxPar PBS), gently vortex, and incubate at room temperature for 0h 30m 0s under low-speed vortexing (use tape to secure the tube in an upright position on the vortex).
In the meantime, prepare the intracellular antibody staining cocktail. This requires individual antibody optimisation and titration. An example is provided below, with emphasis on the use of non-overlapping metals (also, checks against intracellular targets). The final volume should be 50µL, with CSB as the diluent.
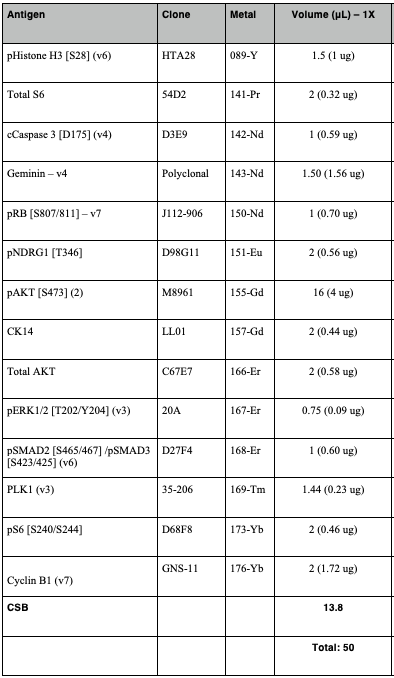
Once the previous step has completed, add 1mL CSB to the suspension and centrifuge in a benchtop centrifuge at1500x g
Discard the supernatant and place the cells on ice °C for 0h 1m 0s.
Resuspend the cells in 200µL ice-cold 50% methanol (diluted in MaxPar PBS and stored at -20°C until use), gently vortex and incubate for 0h 10m 0s at °C on ice.
Once the previous step has completed, add 1mL CSB to the suspension and centrifuge in a benchtop centrifuge at1500x g
Discard the supernatant and repeat the previous step, taking care to discard all the supernatant afterwards (NB: residual supernatant can affect the subsequent intracellular antibody staining).
Intracellular antibody staining
Add the 50µL intracellular antibody cocktail to the cell pellet.
Incubate for 0h 30m 0s with continuous vortexing at low speed (use tape to secure the tube in an upright position on the vortex)
Once the previous step has completed, add 1mL, CSB to the suspension and centrifuge in a benchtop centrifuge at1500x g
Discard the supernatant and repeat the previous step. Remove the supernatant.
During the centrifugation, prepare a fresh 1.6% formaldehyde solution with MaxPar PBS as the diluent.
Antibody fixation & Intercalation
Gently vortex the cell pellet, then add 200µL fresh 1.6 % fresh formaldehyde solution, vortex gently and incubate for 0h 10m 0s at room temperature.
NB: including this fix step increases debar coding efficiency and stained cells can be kept in the fridge for up to 2 weeks and no longer.
Once the previous step has completed, add 1mL, CSB to the suspension and centrifuge in a benchtop centrifuge at 2000x g
NB: the higher centrifugation speed is needed at this point as pelleting is not as efficient after the previous step.
During the centrifugation, dilute 1µL Intercalator (191-Ir & 193-Ir) in 1mL Fix & Perm Buffer.
Discard the supernatant and resuspend the cell pellet in 500µL of diluted Intercalator, gently vortex and incubate for 1h 0m 0s at room temperature or leave overnight in the fridge at 4C (close lid tightly!).
NB: cells can be left at 4C in the Intercalator for up to 72h 0m 0s, however, staining intensity may decrease.
Prepare for acquisition
Without removing the supernatant, add 1mL CSB to the cell-Intercalator suspension and centrifuge in a benchtop centrifuge at 2000x g
Remove the supernatant, taking care not to dislodge the pellet (repeat centrifugation if that is the case), then add 2x 500µL MaxPar CAS+ supplemented with EDTA for 2 mM final concentration (e.g. 4µL of 0.5 M EDTA to 1mL CAS+ solution); in rounds of 2x 500µL transfer the solution to a FACS tube with a 0.35 µm cell strainer lid.
Thoroughly vortex the EQ6 Beads, then add 1:5 beads to cell suspension solution (e.g., 250µL EQ6 beads to 1mL cell suspension solution).
Proceed with sample acquisition on the XT, taking care to specify the correct combination of metals in the template.