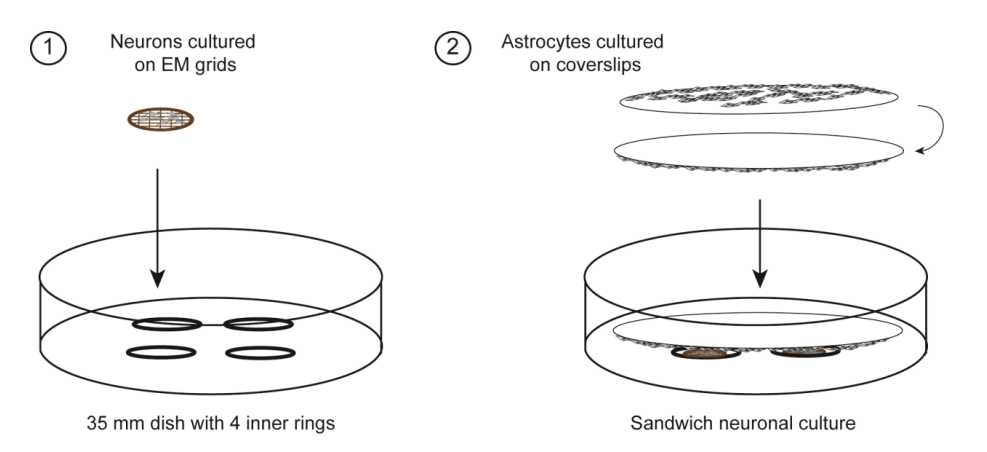
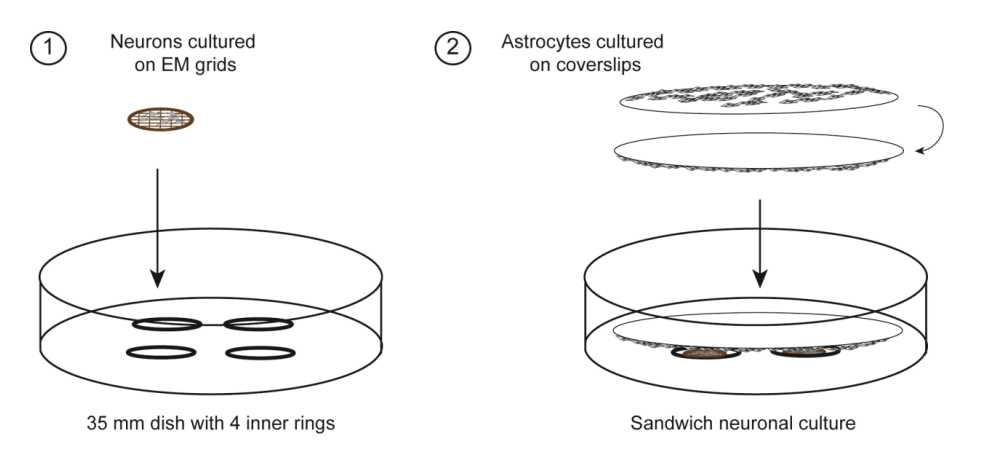
Preparing primary sandwich hippocampal neuron cultures for cryo-electron tomography
Anna Siegert, Arsen Petrovic, Thanh Thao Do, Florelle Domart, Rubén Fernández-Busnadiego, Thomas Dresbach
Abstract
Primary sandwich hippocampal neuron cultures are adapted from the Kaech and Banker protocol (Kaech and Banker, 2006) and provide neuronal cultures with almost no glial cells, which may facilitate the targeting of neurons for cryo-electron tomography (see accompanying protocol by Siegert, Petrovic, Do et al.). In addition, the cells can be seeded at a very low density.
Attachments
Steps
Preparation of the EM grids
For preparation of EM grids, follow the accompanying protocol by Siegert, Petrovic, Do et al.
Preparation of primary sandwich hippocampal neuron culture on EM grids - Astrocyte feeder layer
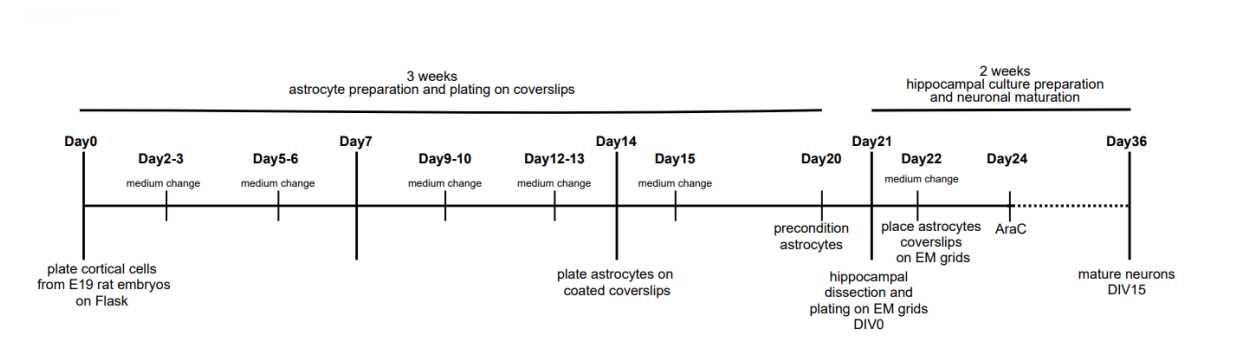
Plate 5 million cortical cells from an E19 rat embryo cortical suspension in T75 flask in MEM10%HS.
Change the medium of the astrocytes twice a week with fresh MEM10%HS preequilibrated at 37°C and 5 % CO2. Slam the flask against the bench surface before aspirating the medium to remove the microglial cells. Two weeks after the dissection, the astrocyte cultures should be more than 50 % confluent.
6-7 days before the hippocampal dissection (2 weeks after the cortical dissection; Fig.1), coat 25 mm sterile coverslips with 0.1mg/mL poly-L-lysine for 0h 15m 0s at Room temperature.
Wash the coverslips 3 times with sterile water and replace the water with MEM10%HS.
Harvest the astrocytes from the flask: Slam the flask and aspirate the medium.
Quickly wash the flask with 0.05 % Trypsin/0.02 % EDTA.
Add 2mL of trypsin/EDTA and incubate 0h 2m 0s at 37°C until the cells detach.
Stop the trypsination by adding 5mL of MEM10%HS.
Release the cells by multiple rounds of pipetting with a 10 mL serological pipette.
Transfer the cells to a 15 mL sterile tube and centrifuge for 0h 5m 0s at 500x g,0h 0m 0s.
Resuspend the cells in 2mL of MEM10%HS complete medium.
Count the cells and plate them dropwise on each of the coverslips: use 200,000 cells in a total volume of 100µL of medium per coverslip.
One day after plating, change the medium of the astrocytes again with fresh preequilibrated MEM10%HS.
Preparation of primary sandwich hippocampal neuron culture on EM grids - Primary neuron cultures with astrocyte feeder layer
One day before the hippocampal dissection, precondition the astrocyte feeder layer in Neurobasal medium + 2 % B27 + 2millimolar (mM) L-Glutamine pre-incubated at 37°C and 5 % CO2 for few hours.
Dilute the hippocampal suspension to reach a density of 200,000 to 350,000 cells per mL in DMEM10%FCS.
After two hours, add 500µL of pre-heated DMEM10%FCS per dish.
Incubate at 37°C and 5 % CO2.
Replace the plating medium with 2mL of pre-conditioned Neurobasal medium from the astrocyte feeder layers’ dish.
To stop the proliferation of glial cells on the grids, treat the primary neuron cultures with the anti mitotic agent arabinofuranoside (cytosine-β-arabinofuranoside hydrochloride) three days after plating the hippocampal cells.
Add arabinofuranoside to a final concentration of 2.45micromolar (µM) to each dish and distribute evenly by gently moving the dish in a circular motion.
The neurons are considered mature starting from 15 days in vitro (DIV15).