Plate Protein Expression on Autoinduction media
Stephane Fadanka, Chiara Gandini
Abstract
The current protocol describes the preparation and use of 2X YT autoinduction medium for recombinant protein expression on Petri dishes. This protocol allows for reproducible and time effective expression experiments to be undertaken with minimal user intervention as compared to standard procedures using IPTG.
Steps
Preparation of the Overnight Pre-inoculum
Grow culture 10mL of desired bacteria strain in LB broth supplemented with the appropriate antibiotic, grow overnight at 37C37°C .
Preparation of Auto-induction media
Prepare the needed amount of 4xYT autoinduction agar medium
- ( It is recommended to prepare and use Auto induction media the same day, make sure to prepare just the amount needed for the experiment) e.g 20ml of Autoinduction in 9cm Petri plates).
Composition:
4x YT (0.5L in 1L bottle) liquid
6g Na2HPO4
3g KH2PO4
20g Tryptone
5g Yeast Extract
5g NaCl
Weigh and dissolve all powders and salts in 0.5L of distilled water and transfer to a1L Duran bottle.
Prepare 50% glycerol, 10% glucose and 5% lactose solutions for reconstitution of the autoinduction media after sterilisation:
50% (vol/vol) glycerol
-
- Measure 50ml of glycerol in a 250ml bottle
-
- Add 50 ml of distilled water and mix by shaking.
10% (weight/volume) glucose
-
- Weight 10g of glucose
-
- Dissolve in 100ml of water
5% lactose
-
- Weight 10g of lactose
-
- Dissolve in 200ml of water
Enough distilled water to dilute and reconstitute media after sterilisation (1L)
Sterilize all solutions by autoclaving.
After reconstituting the media, add an appropriate antibiotic and pour 20mL of reconstituted media in 9cm Petri dishes.
- (Carefully determine the number of plates needed and prepare the volume of media to prepare accordingly make sure to prepare 3 plates per culture and to include replicates for the control as well).
Allow the plates to solidify for about0h 30m 0s
After Autoclaving, reconstitute autoinduction medium
Inoculate the plates with 0.2mL of prepared overnight culture using the spreading method.
Incubate the plate overnight at 37°C.
Collection of Cell Biomass
After incubation, period, check cell growth and collect Biomass from each plate using a scalpel blade or any other available utensil enabling to scrap the surface of the plate without carrying the gel.
- Cell Biomass can be collected and stored in a suitable container (1.5ml Eppendorf tubes; 15-50ml Falcon tubes.. etc.) and stored at
-20°Cor-80°C.
Functionality Testing of Expressed Protein
Successful expression and functionality of the produced enzyme were evaluated the next day by SDS PAGE and PCR.
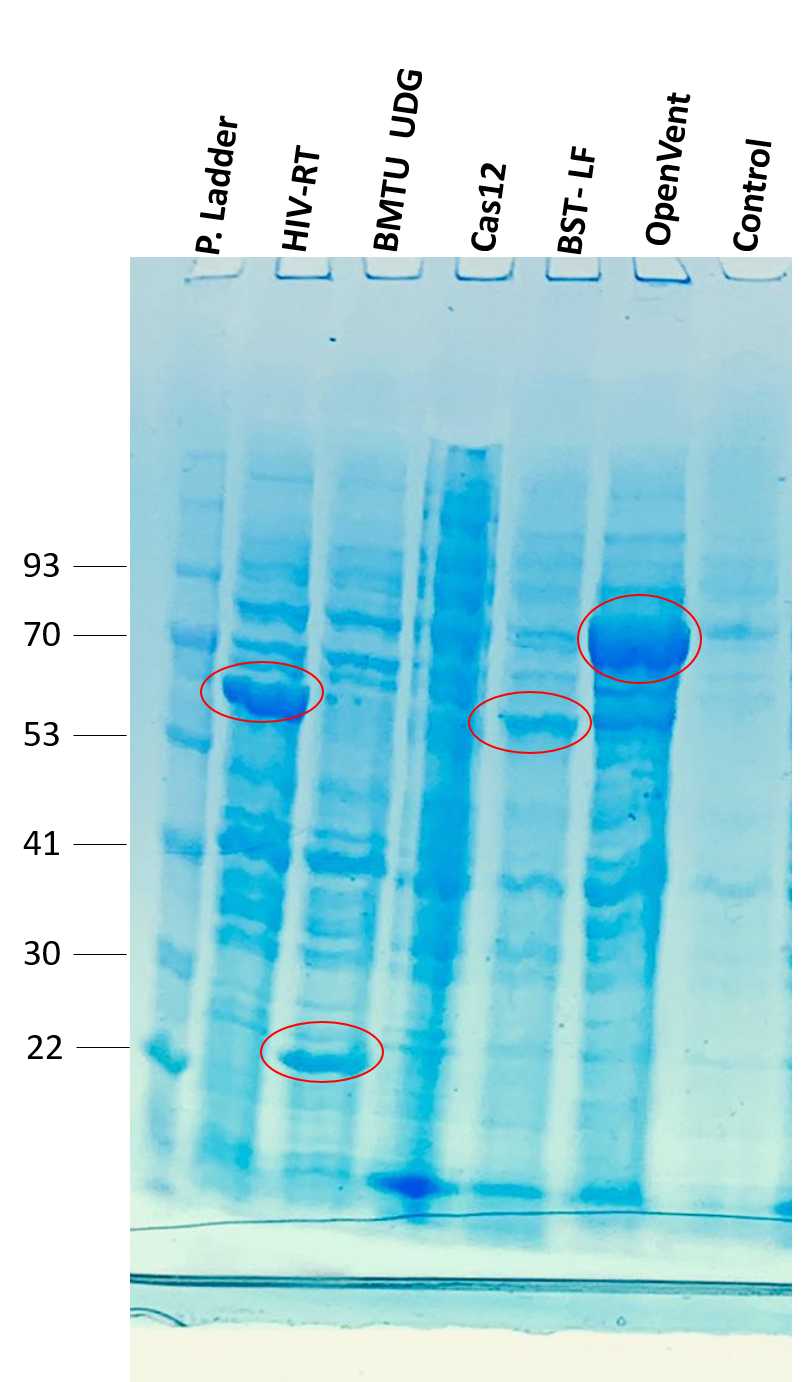
use a small fraction of stored cell pellet to run an SDS PAGE and check if the protein were successfully expressed:
following results were obtained using NuPage Pre-cast gel, MOPs sample Buffer and 2X SDS sample preparation buffer.
- Various enzymes were expressed using prepared auto-induction media and successful enzyme expression was confirmed by the presence of bands at the expected size on the gel.
The functionality of produced enzymes was assessed for OpenVent DNA Polymerase by carrying out a PCR reaction using cellular reagents preparations produced with an in-house enzyme:
Preparation of Cellular reagents from Plate Auto-induction media
1. Transfert a small lump of cell biomass into a fresh tube and freeze the rest at -20C. 2. Resuspend the cells into 1.2ml of Cold PBS and follow the original protocol for cellular reagents preparation from **step6.2.9** ([Protocol here](https://docs.google.com/document/d/1mf5weGcWcsFRUcoyyZnwkNkb2xEQZQUkSUpYNFrKX7g/edit)). 3. Dilute resuspended cell pellet into cold PBS to obtain a suspension of A600 between 6.5 and 8 _Measure A600 of a neat, 1:10 or 1:100 dilution. Multiply the value to get the actual final A600 number._ You might _have to dilute several_ times _before you get the right OD._ 4. Calculate the volume of your final cell suspension to aliquot in each PCR tube (that would contain 2 x 10<sup>8</sup> cells), using the equation: **Volume to** Aliquot **= 200/final A600 of cell suspension.** e.g. if your final A600 is 6.5, then - volume containing 2x10<sup>8</sup> cells = 200/6.5 - volume containing 2x10<sup>8</sup> cells = 31µl 5. Aliquot either single reaction or 10X reactions worth of cellular reagents into 8-tube strips of 0.2 ml PCR tubes e.g. using the example above, 3.1 µl (1x reaction) or 31 µl (10x); 6. Label tubes with reagent, date and operator 7. Incubate the tubes at 60C for 10min in a thermocycler or Heat block (heat treatment) to make sure that produced cellular reagents are free from any living bacteria. 8. Place the tube strips with aliquoted cellular reagents carefully in a container 1/2 filled with desiccant, leave tubes opened (using vacuum Tupperware is ideal). 9. Place the container overnight in a 37 °C static incubator. 10. After 18-24hrs check to see if the cellular reagents are completely dry. _Note: Leaving the reagents longer at 37 °C should not hurt their efficacy._ 11. Once dry, close the lids and place them in a small bag at +4°C with a small amount of desiccant.
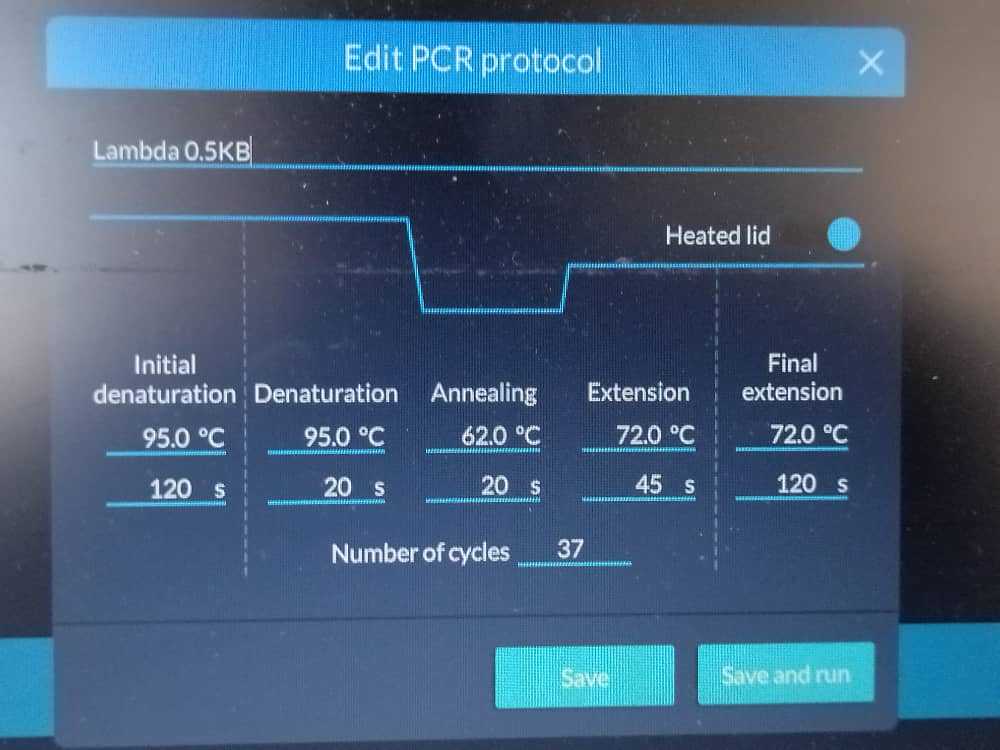
A PCR reaction was carried out using prepared cellular reagents for OpenVent DNA Polymerases using Lambda genome template 0.5kb.
PCR conditions:
T annealing 62 C, extension 72C for 45s , 37 cycles, 25ul total volume using 1ul of OpenVent, template:lambda genome 1ul of a 1:10 dilution (50ng/ul concentration of template), primers to amplify 0.5 kb.
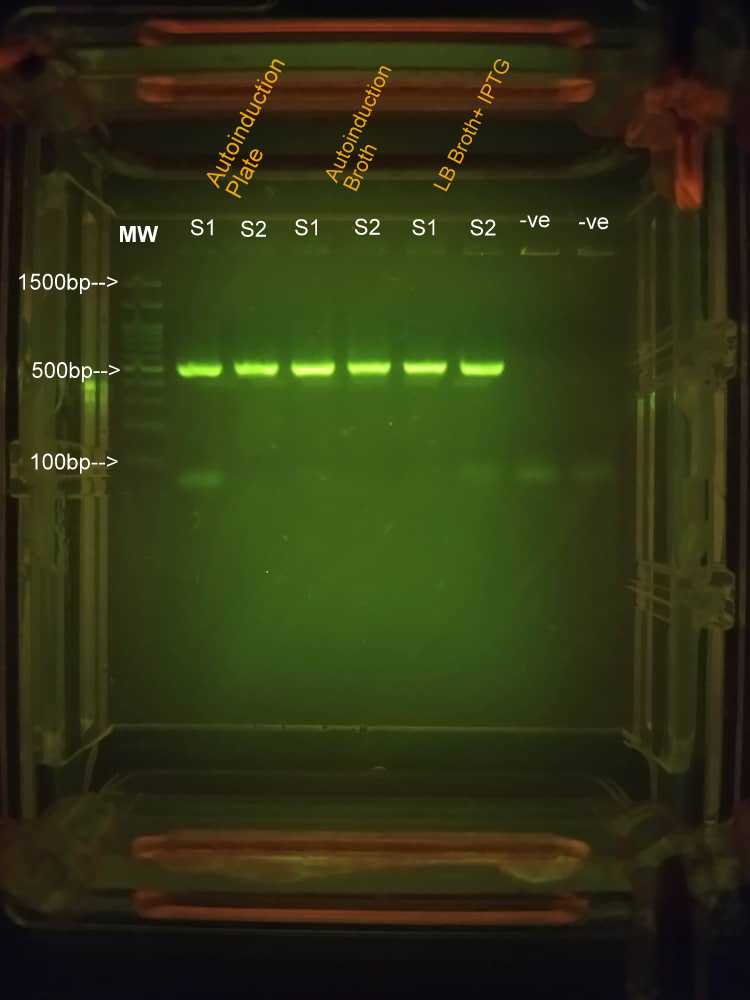
PCR Results were visualised via Agarose gel electrophoresis on 1.5% agarose gel using TBE buffer system, with 9ul of each amplicon loaded;
Bands of expected sizes were spotted after running the gel.