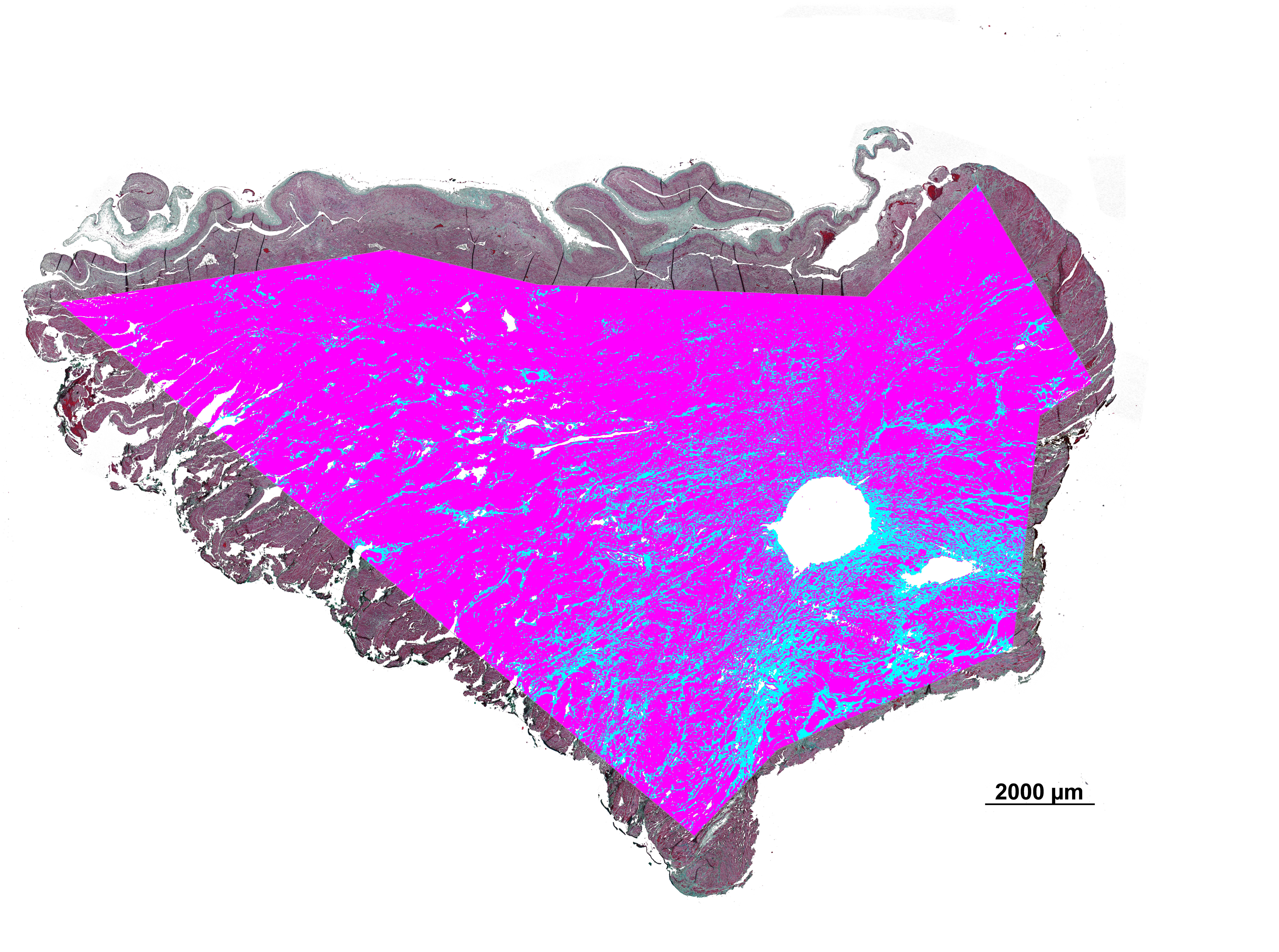
Optimized protocol for the RNA isolation of laser microdissected formalin-fixed paraffin-embedded uterine scar tissues for RNA expression analyses
Alexander Paping, Clara Basler, Rebecca C. Rancourt, Loreen Ehrlich, Kerstin Melchior, Wolfgang Henrich, Thorsten Braun
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Samples for histological analyses are often formalin-fixed paraffin-embedded (FFPE) and slide-mounted. This complicates RNA extraction for molecular downstream applications. Additionally, when the region of interest is limited to a smaller area with laser microdissection (LMD), extracting adequate quality and quantity RNA is difficult. This is a protocol for maximized RNA output from FFPE tissue devised to identify and analyze gene expression of human maternal uterine scar tissue obtained from uterotomy scars resulting from prior cesarean deliveries. Gomori Trichrome staining allowed for region identification for LMD. Successful RNA isolation, reverse transcription, and quantitative real-time polymerase chain reaction (qRT-PCR) were performed. This description provides an optimized step-by-step protocol that yields sufficient RNA for qRT-PCR analyses from challenging tissue such as LMD-FFPE samples.
Steps
Step 1: Sample Collection
During cesarean delivery: Collect sample of the uterine wall from the lower uterine segment (after delivery of the baby and before delivery of the placenta).
Find scarred area in lower uterine segment (best visualized with intraoperative ultrasound when uterus is exposed just before childbirth. Mark the scarred area e.g. with cauter points. Place the uterotomy 1 cm above the identified scar).
Cut out small piece of uterine tissue containing healthy myometrium and scarred area after child is born and before intravenous oxytocin application.
Immediately continue with tissue processing.
Step 2: Tissue Processing
Treat surfaces and eqipment with RNase Away to prevent RNA degradation by RNases during tissue preparation.
Cut samples immediatly after collection to an adequate size (no thicker than 1 cm) to allow formalin and paraffin penetration (and thus fixation) of the entire piece of tissue.
Put sample into 4% neutral buffered formalin in an adequate sized vessel ensuring constant contact of tissue and formalin for 24-48 hours at room temperature.
Step 3: Paraffin Embedding
Dehydration and Paraffinization in Citadel 1000
| A | B |
|---|---|
| 70% Ethanol | 3 hours |
| 70% Ethanol | 1 hour |
| 70% Ethanol | 1 hour |
| 80% Ethanol | 1 hour |
| 96% Ethanol | 1 hour |
| 100% Ethanol | 1 hour |
| 100% Ethanol | 1 hour |
| 100% Ethanol | 1 hour |
| Chloroform | 1 hour |
| Chloroform | 2 hours |
| Paraffin | 2 hours |
| Paraffin | 3 hours |
Paraffin Embedding in Histocentre 2
Step 4: Sectioning of Tissue Blocks with Microtome
Before cutting, cool tissue blocks at 4°C for 1 hour to harden the wax and thus allow easier cutting.
Heat water bath with deionized water of the rotary microtome to 36°C.
Fasten cooled block into block holder of the rotary microtome.
Cut 5 µm thick slides.
Mount unwrinkled sections on Superfrost Plus Langenbrick glass slides.
Dry sections overnight at 37°C in heating cabinet (Heraeus Type 290).
Store slides at room temperature protected from light until further use.
Step 5: Gomori Trichrome Staining
| A | B | C |
|---|---|---|
| Deparaffinization and Rehydration | ||
| Xylol | 5 min | |
| Xylol | 5 min | |
| 96% Ethanol | 2 min | |
| 70% Ethanol | 2 min | |
| 50% Ethanol | 2 min | |
| Aqua dest. | 2 min | |
| Staining | ||
| Boin Solution | 30 min | 56°C |
| Running tab water | 5 min | |
| Weigert's hematoxylin | 10 min | |
| Running tab water | 10 min | |
| Trichrome Solution | 25 min | |
| 0.5% acetic acid | 2 min | |
| 0.5% acetic acid | 1 min | |
| Dehydration | ||
| 96% Ethanol | 1 min | |
| 1. 100% Ethanol | 2 min | |
| 2. 100% Ethanol | 2 min | |
| Xylol | 5 min | |
| Xylol | 5 min | |
| Put on cover glass |
Step 6: Hematoxylin Eosin Staining for LMD
Stain the sections according to a short hematoxylin eosin protocol to identify regions of interest for later LMD use.
Diethylpyrocarbonate (DEPC) treat alcohols and water used in this protocol and carry out final dehydrating steps with undenatured ethanol.
0.1% DEPC Solution:
999 ml deionized water + 1 ml DEPC
Mix overnight
Autoclave in the morning
| A | B |
|---|---|
| Deparaffinization and Rehydration | |
| 1. Xylol | 20 seconds |
| 2. Xylol | 20 seconds |
| 3. Xylol | 20 seconds |
| 1. 100% Ethanol | 30 seconds |
| 2. 100% Ethanol | 30 seconds |
| 1. 95% Ethanol | 30 seconds |
| 2. 95% Ethanol | 30 seconds |
| 1. 70% Ethanol | 30 seconds |
| 2. 70% Ethanol | 30 seconds |
| Aqua dest. DEPC | 30 seconds |
| Staining | |
| Hematoxylin (Harris, Merck) | 60 seconds |
| Aqua dest. DEPC | 30 seconds |
| Eosin Y Solution (Sigma) | 10 seconds |
| Dehydration | |
| 70% Ethanol | 30 seconds |
| 95% Ethanol | 30 seconds |
| 100% Ethanol | 30 seconds |
Let sections air-dry and do not put on a cover glass.
Immediatly transfer stained slide to laser microscope or store in a sterile falcon tube at 4°C.
Do not freeze- as thawing causes water condensation on the slide which actives RNases.
Step 7: Laser Microdissection and Scratch off
Put slide into the slide holder with the specimen facing downwards.
Encircle the area of interest with the freehand point to point function.
Calculate size of the area (µm2 * 5µm thickness) and expected RNA amount
Transfer slide with laser microdissected sample to a workbench (which has been previously decontaminated with RNA Zap).
Prepare a 1.5 ml Eppendorf Tube with 150µl Proteinase K Buffer (PKD, supplied by RNeasy Kit) and 1µl Stabilizer Reagent.
Transfer the collected scratched off sample into the tube containing buffer.
At this point, the tube containing the collected LMD samples in buffer can be stored at 4°C for up to 24 hours
Once enough tissue has been collected, freeze the sample at -20°C at least briefly and thaw it again right before the RNA extraction.
Step 8: RNA Extraction (Adapted Qiagen FFPE RNeasy Kit Protocol)
Thaw Scratch Sample with PKD Buffer.
Add 10µl of Proteinase K. Mix carefully. Do not vortex!
Incubate at 56°C in Eppendorf Thermomixer comfort (with shaking function) overnight.
Let sample rest at room temperature until heating block has reached 80°C
Incubate sample at 80°C for 15 minutes in Thermomixer
Cool on ice for 3 min.
Centrifuge for 15 min at 20,000 x g (13,500 rpm).
Transfer the supernatant to a new 1.5 ml tube without disturbing the pellet.
Add DNase Booster Buffer:
approximately 16 μl = tenth of the total sample volume.
Add 10 μl DNase I stock solution. Mix carefully. Do not vortex!
Incubate at room temperature for 15 min. Flick tube from time to time (approx. every 3 minutes).
Add 320 μl Buffer RBC and mix thoroughly.
Add 720 μl ethanol (100%) and mix carefully but thoroughly.
Transfer 650 μl of the sample to a supplied RNeasy MinElute spin column in a 2 ml tube.
Centrifuge for 15 s at 8000 x g (10,000 rpm).
Discard the flowthrough.
Transfer the rest of the sample to the same RNeasy spin column and centrifuge for another 15 s at 10 000 rpm. Discard the flowthrough.
Add 500 μl Buffer RPE to the RNeasy MinElute spin column. Centrifuge for 15 s at 8000 x g (P10,000 rpm) to wash the spin column membrane. Discard the flowthrough.
Add 500 μl Buffer RPE to the RNeasy MinElute spin column. Close lid, centrifuge for 2 min at 8000 x g (10,000 rpm) to wash the spin column membrane.
Discard the flowthrough and the collection tube.
Place the RNeasy MinElute spin column in a new 2 ml collection tube. Open the lid
of the spin column, and centrifuge at full speed for 5 min. Discard the collection tube with the
flow-through.
Place the RNeasy MinElute spin column in a new 1.5 ml tube. Add
14–30 μl RNase-free water directly to the spin column membrane. Close the lid gently, and
centrifuge for 1 min at full speed to elute the RNA.
Step 9: Control of RNA Integrity (Agilent 2100 Bioanalyzer, Nano Kit)
Prepare Ladder
Heat in RNAse free Eppi for 2 minutes at 70°C
Aliquot and store at -80°C
Prepare gel
Centrifuge 550µl RNA-gel-matrix on spin filter for 10 minutes at 1500 rcf
aliquot 65µl Gel and store at 4°C for up to four weeks
vortex blue RNA dye and centrifuge
add 1µl RNA dye to 65µl gel
vortex dye gel mix and centrifuge for 10 minutes at 13 000 rcf
Load chip
Place the chip on the priming station
Run the chip immediatly (within 5 minutes)
Apply 9µl of gel-dye mix to the G-well
Position the plunger at the level of 1mL and close the priming station
Press down the syringe until it is held by the clip → wait 30s → then loosen the clip and hold for 5s (note: hold the syringe well as it moves back to its original position due to the negative pressure) → slowly draw back to 1mL
Open the priming station and apply 9 µl of gel-dye mix to each of the two G-wells with a white background
Apply 5µl of RNA marker (marked green) to all 12 sample wells and the ladder well
Apply 1µl of ladder to ladder well
Transfer 1µl of RNA sample to each of the 12 sample wells. Pipette 1 µl of RNA marker into free wells without samples
Vortex the chip at 2400 rpm for 1 min
Clean before use:
-
Electrode Cleaner "RNase ZAP": Pipette 350 µl RNase ZAP-water mixture (1:1) into a well, insert, close the flap, wait 1 minute and remove
-
Electrode Cleaner "Water": Apply 350µl RNase-free water to a well, close the flap and wait 10s. Then take it out and let the water evaporate for another 10s with the flap open.
Measure in Bio-Analyzer
Open the software 2100 Expert
Select Eukaryote Total RNA Nano protocol
Insert the chip, enter the sample description in the table and start the measurement (takes about 30 minutes)
Clean with H2O after measurement
Step 10: cDNA Synthesis (cDNA synthesis Kit BioRad protocol)
Use a Eppendorf Mastercycler for Reverse Transcription of up to 1µg RNA to cDNA in a 20µl volume with the BioRad iScript Synthesis Kit and protocol
Set up a Sample-RT Control with RNA template and primer mix but no RT
Set up NTC control without RNA template but with RT and primer mix
Use 0.2 Tube Stripes
4µl iScript primer mix of oligo dt and random primers. With this primer mix as many RNA fragments as possible can be detected and transcribed
1µl of Reverse Transcriptase
up to 15µl of RNA elute corresponding to 1µg of RNA
add up to 20µl with Nuclease free water
Centrifuge
Start BioRad protocol in Mastercycler
Priming step: 5 minutes at 25°C
Reverse transcription step: 20 minutes at 46°C
Inactivation of RT Step: 1 minute at 95°C
Cool down and hold at 4°C
You receive 20µl of cDNA eluate with a concentration of 50 ng/µl
Freeze at -20°C
Step 11: qPCR (TaqMan techc)
Carry out qPCR in Singleplex using Taqman probe technology in 96 well plates in an ABI 7500 (Applied Biosystems)
Test samples in triplicates
Run interplate calibrator on each plate
Load plate according to sample maximization method: One gene per plate and maximum amount of samples
Load wells:
- Taqman Universal PCR Mastermix (2X)
- Taqman Gene Expression Assay (10X)
- cDNA (<100ng)
- nuclease free water
Protocol:
| A | B | C | D |
|---|---|---|---|
| enzyme activation | 10 min | 95°C | |
| denaturation | 15 sec | 95°C | 45 cycles |
| annealing + elongation | 1 min | 60°C |
Step 12: Gel electrophoresis
Prepare TAE Buffer (1X):
Preparation of 1000 ml TAE (1X): 20 ml TAE (50X) + 980 ml H2O
Prepare Agarose gel (5%):
Weigh 5g agarose in 100 ml 1xTAE buffer and carefully swirl in the Erlenmeyer flask
dissolve in microwave at 560W (boil until bubbles appear)
Let it cool down for approx. 10 minutes (swirl carefully) to 50-60 ° C
Solution should now be clear (otherwise boil again)
Add 10µl ethydium bromide (1µl per 10mL gel) to the cooled solution (do not boil)
Stir with a stir bar or by swirling -> liquid gel
Pour out the gel, make sure that there are no air bubbles in it (if necessary, use a pipette tip to remove it)
Insert the comb (0.5-1cm away from the upper edge)
Let it harden for approx. 1 hour until it is slightly milky → pour TAE Buffer (1X) over it → remove comb
Electrophoresis
Load the first pocket with 6µl of the dna ladder (marker) (should have the same volume as samples)
Load further pockets with 6µl mixture of 5µl PCR sample and 1µl loading buffer
Let it run for approx. 60 min at xxV (5 volts per cm of gel length)
Analyze under UV light