OT-2 PCR sample preparation protocol
Ana Mariya Anhel, Lorea Alejaldre, Ángel Goñi-Moreno, Manuel Gimenez
Disclaimer
Abstract
This protocol is meant to perform samples preparation of PCR plates for 1 or more primer sets (defined number of primers per set established by the user) to the same samples, i.e, we will have for all the selected samples (we can set the initial well and number of samples) of all source plates the master mix with different primer sets (combination of primers, water and polymerase mix) in the final plates.
To run this protocol is needed a python script for an Opentrons 2 robot and an excel (.xlsx) file with several variables making the protocol modular to reactives, volumes of transfer, type of plates, number of primers per set, etc.
In our laboratory, this protocol has been used as part of the "High-throughput workflow for the genotypic characterization of transposon library variants" also available in protocols.io to prepare PCR samples for sending to sequencing and for checking the correct introduction of the transposon in the bacterial genome.
This protocol is a set of instructions or description of the LAP repository entry LAP-PCR-OT2-1.0.0
Before start
Steps
Files Preparation
Preparing Customized Template
Preparing the template (a .xlsx) with the specific variables for each experiment.
Here we attach one Excel with several sheets and a PDF file explaining each variable:
- GeneralVariables: variables related mainly to the labware that is going to be used
- SamplesPlateVariables: variables related to the specifications of each source plate
- PipetteVariables: variables related to the pipette(s) that are going to be used
- ReagentsPerReaction: variables that will determine the final mix of the wells
- ModuleVariables: variables related to the modules used in the protocol, the thermocycler and the heater-shaker
- TemperatureProfile (Optional): PCR program that will be performed in the thermocycler if set as True in the ModuleVariables sheet
- Maps (Optional): sheet(s) with the names of the samples in the source plates and will be reflected in the final plate map(s) --> not included in the template but needed to be included if the variable Map IDs has a value .
Fill the template with the corresponding values
Store it with the name VariablesPCR.xlsx
Transferring file to Robot
Transfer the VariablesPCR.xlsx to the directory /data/user_storage of the OT robot that we will use to perform the protocol.
Here, we present a summary of how to transfer the files in 3 Operative Systems: Windows, MacOS and Linux .
MacOS/Linux
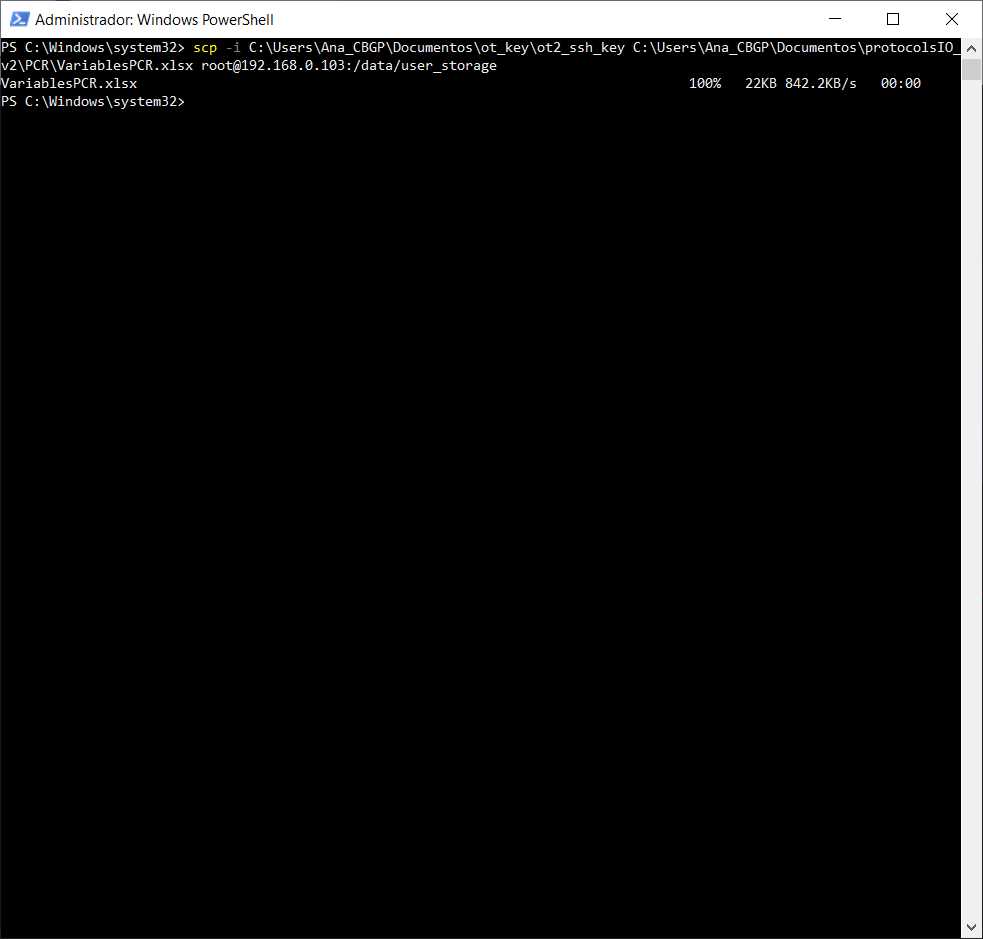
We will use the command line with scp to transfer the file VariablesPCR.xlsx to the OT system.
We need to perform the following command
#Passing Files to OT
scp -i [ot_key] [file] root@[IP_OT]:/data/user_storage
#Transferring files to OT (MacOS 13 and 14)scp -Oi [ot_keypath] [file path] root@[OP_robot]:/data/user_storageWindows
There are several ways to pass files from a Windows to a Linux (for example, with a virtual machine or Windows Powershell in the latest versions of Windows).
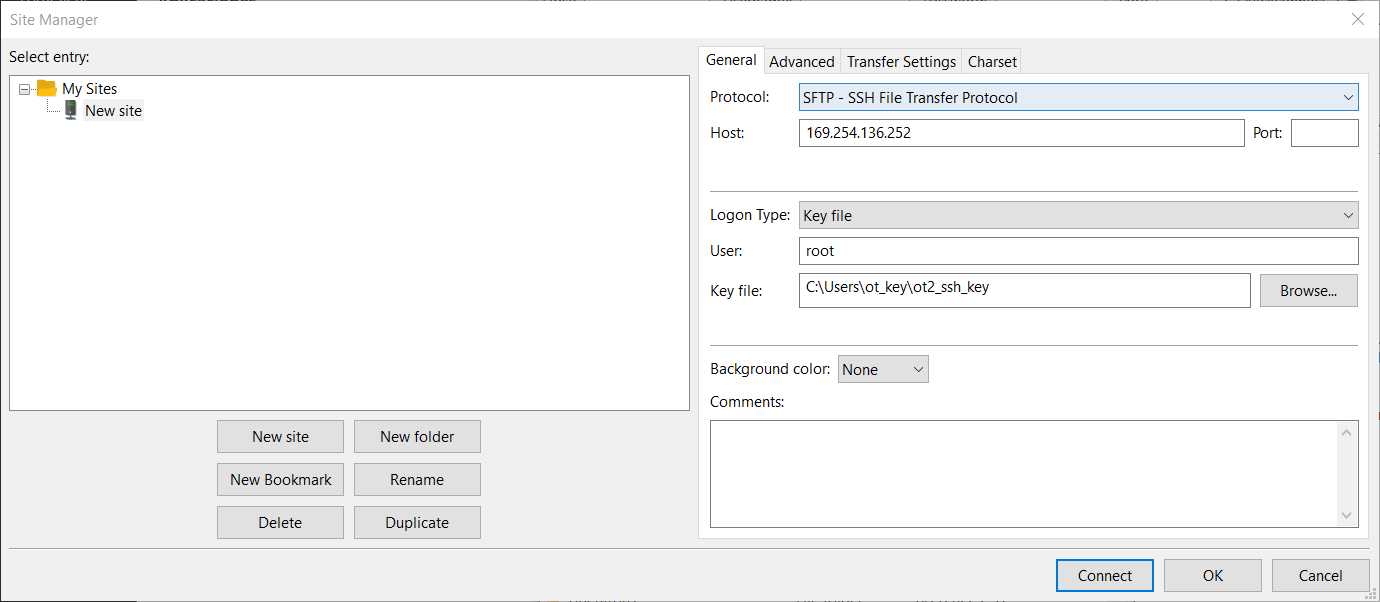
Here, we will use FileZilla (https://filezilla-project.org/download.php?type=client).
Go to File -> Site Manager -> New Site -> Change Protocol to SFTP . Then, introduce in Host the OT IP, change the Logon Type to Windowskey file, change the user name to root and give the directory where the OT key is. It should look something like this
Then press connect, and we will have a connection between our computer and the robot.
After this connection, we should be able to move out VariablesPCR.xlsx (in our computer) to the directory /data/user_storage in the robot.
This method of transferring files can also be done in Linux and MacOS.
Adding the custom labware
There is only a need to do this step when the labware you are using is not OT official or included in the OT app.
Creation of .json file
The description file can be obtained by describing the labware dimensions at https://labware.opentrons.com/create/
Uploading files to the OT App
In the OT app, we need to perform the following route: Labware -> Import -> Choose File -> Select file we have created in step 3.1
Transfer labware files to the robot
If you are using the entry LAP-PCR-OT2-1.0.0 and custom labware ,. an additional step is needed, which is transferring a folder with the custom labware
We need to create for our custom labware a folder with the API name containing the description file (.json) called 1.json and then transfer that folder to the robot's folder /data/labware/v2/custom_definitions/custom_beta in a similar way as in the Step 2 but with the difference that is a directory that needs to be transferred and not a file.
#Transferring the custom labware to OT (Linux)
scp -i [ot_key] -r [directory_custom_labware] root@[IP_OT]:/data/labware/v2/custom_definitions/custom_beta
Prepare Robot OS
Install needed packages
This script needs the package openpyxl , which is not installed by default in the OT-2 robots
Connect to the robot
to find the IP of the robot in which you want to run the script
To connect to the robot, you can do it via ssh with the following command
#Connect to Linux based OT via ssh
ssh -i [path ot_key] root@[Robot_IP]
```In Wsuccessfulindows, you can do this command in Windows Powershell
<Note title="Citation" type="success" ><span>If the connection has been successful you should obtain a screen similar to the following image</span><span></span><img src="https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.n92ldpyznl5b/pd8zbxsu714.jpg" alt="Drawing obtained when entering an OT-2 system" loading="lazy" title="Drawing obtained when entering an OT-2 system"/></Note>
Install the package
Once inside the robot's system,, you need to run the following command
#Install openpyxl package (Linux 4.14.74-v7)
pip install openpyxl
Run protocol
Load script in OT-App
Now that we have transferred the variable files to the robot, we can import the script and run it in the selected robot
Software
| Value | Label |
|---|---|
| Opentrons App | NAME |
| Windows >=10, Mac >=10 | OS_NAME |
| , Ubuntu >=12.04 | OS_VERSION |
| https://opentrons.com/ot-app/ | REPOSITORY |
| Opentrons | DEVELOPER |
Load the script in the OT app
Protocols -> Import -> Drag Python script
Software
| Value | Label |
|---|---|
| LAP Repository | NAME |
| www.laprepo.com | REPOSITORY |
| https://biocomputationlab.com/ | DEVELOPER |
Select Robot to Perform Script
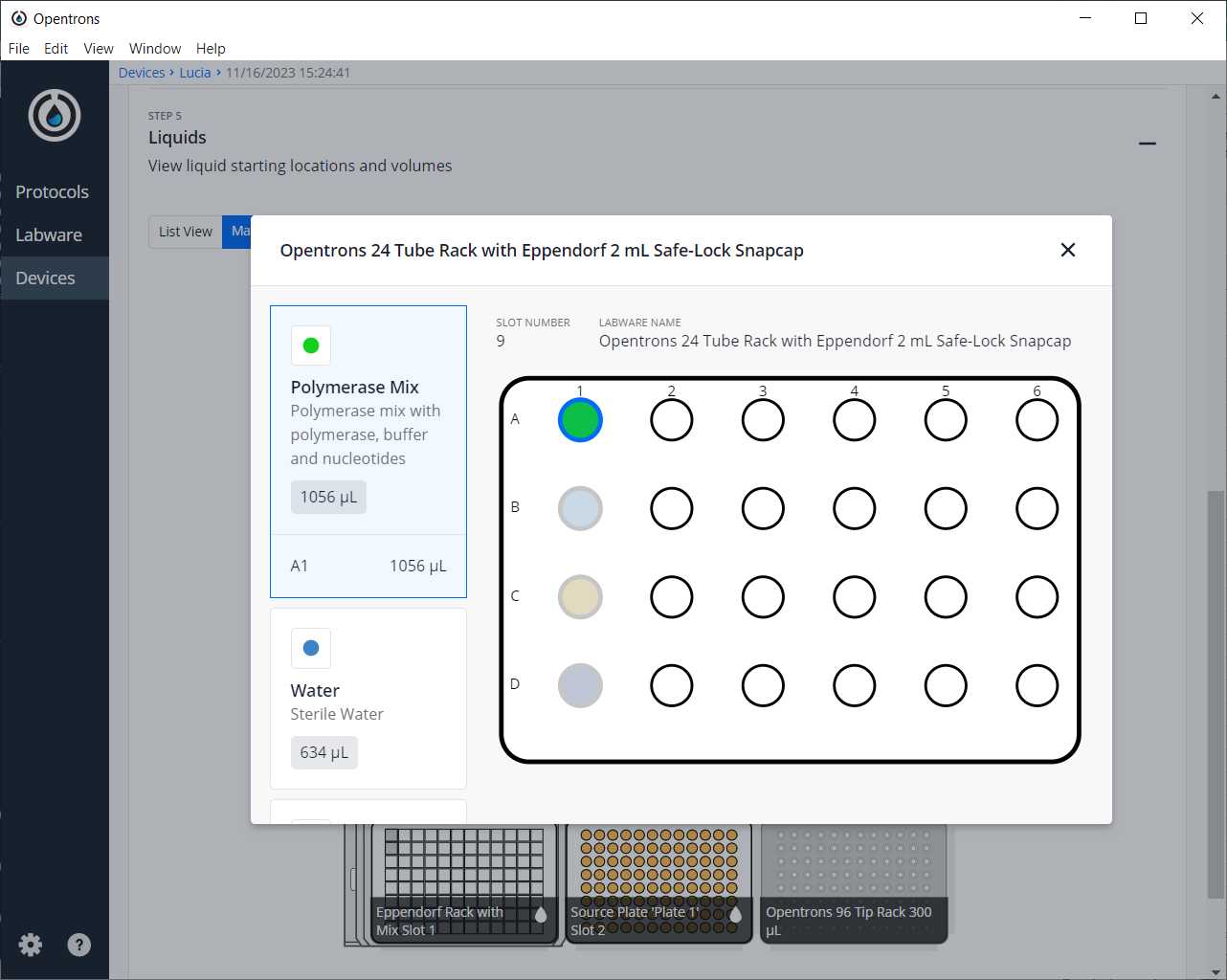
Click in the protocol -> Start setup -> Choose the OT where the file VariablesPCR.xlsx is -> Proceed To Setup
After clicking on Proceed to Setup, you should obtain the labware positions in the Labware tab and the reagents, with their corresponding volume, in the Liquids tab.
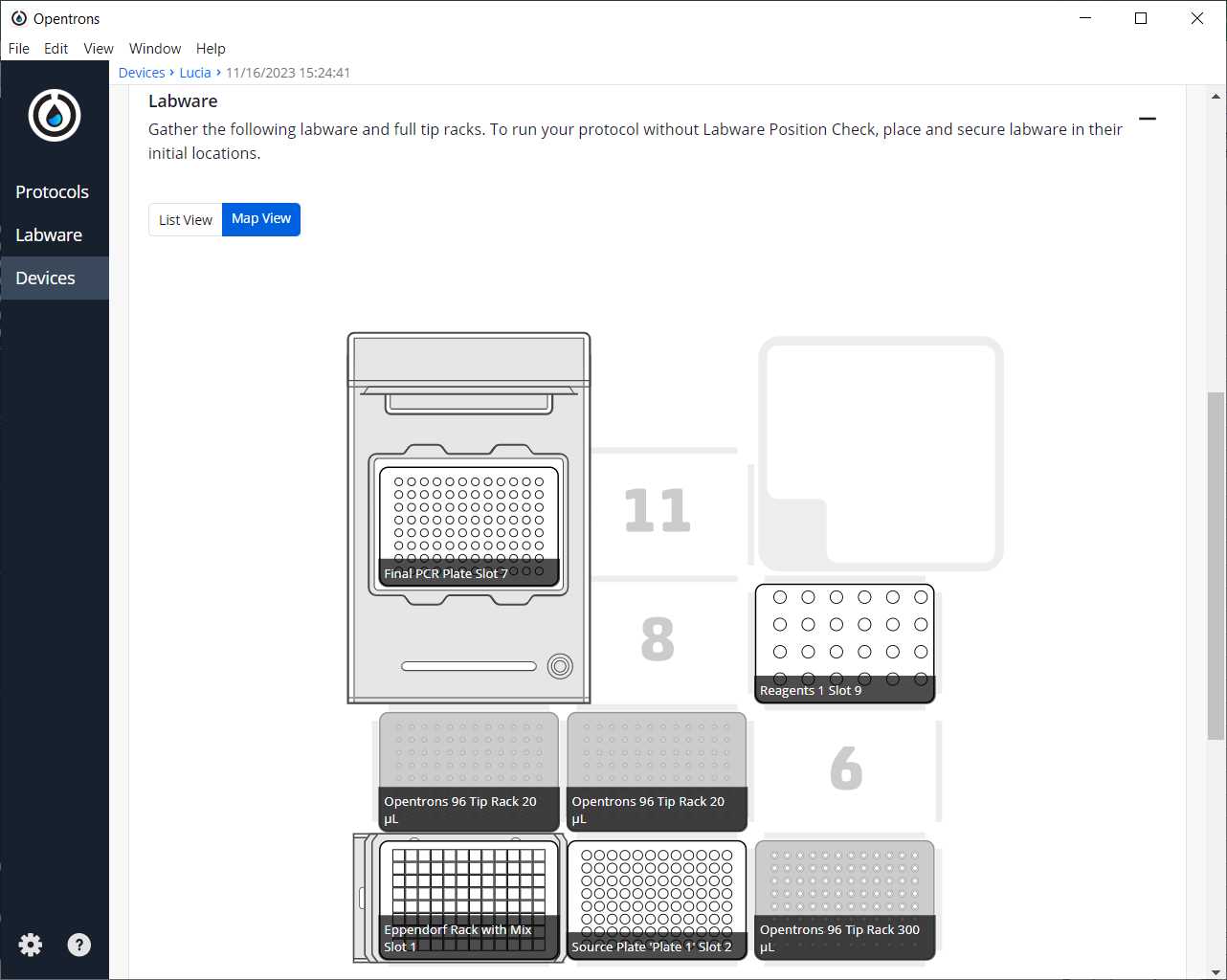
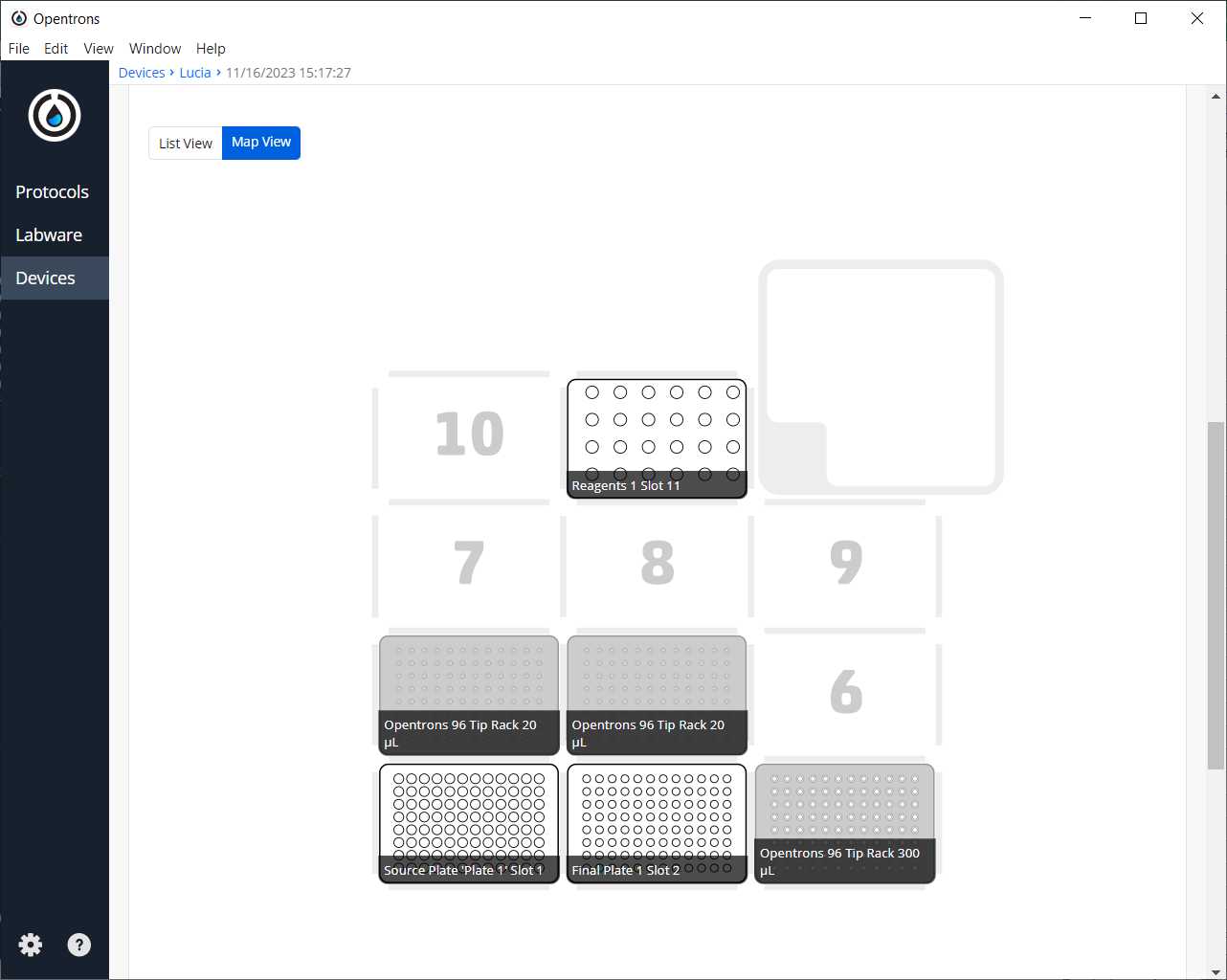
Run Protocol in OT
Make sure the needed calibrations are done
Pipettes, tip racks and tip length calibrations need to be done for the items used in this run
Labware position check is performed (if needed)
Clean the surface of the robot with 70% ethanol to clean and disinfect the surfaces
Set the labware and reagents as shown in the OT-App
Start Run
The procedure that the robot is going to do is mainly divided into 6 parts:
- Creation of mix(es) transferring primers, water and polymerase to new tube(s)
- Mixing with either a pipette or heater-shaker
- Distribute mix to all plates
- Distribute samples to the corresponding wells (as many transferring of 1 sample as the number of primer sets)
- Generate identity maps to be exported
- (Optional) PCR program for thermocycler module
After-running
Retrieve labware from the OT
Importing map from robot
To retrieve the file we can and reproduce it by transferring the files to the computer.
They will be in the directory /data/user_storage . It will be a file with an extension .xlsx and have the name provided in the input variable file
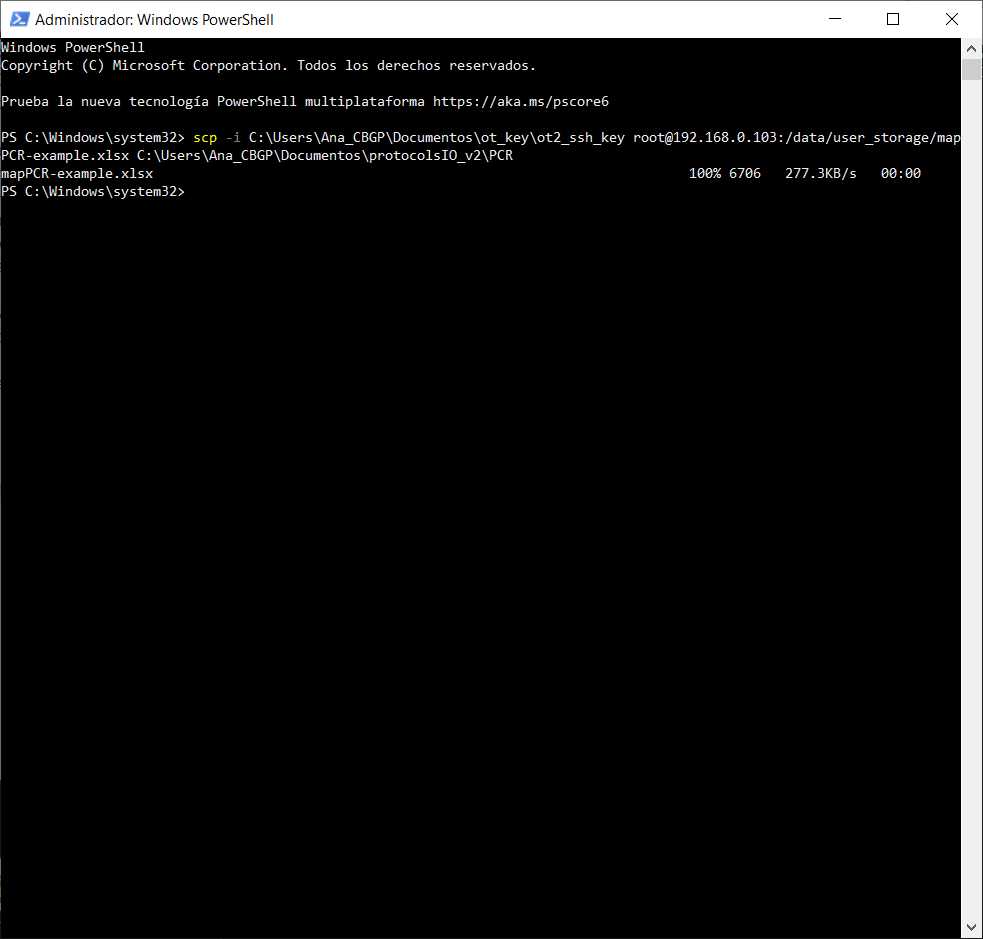
#Transferring files from OT to computer (Linux, macOS)
scp -i [path_ot_key] root@[IP_robot]:/data/user_storage/[name_map].xlsx [ultimate_path_computer]
Example
We want to perform a PCR mix preparation of 94 colonies and 2 controls that are in 2 source plates(40 first colonies + 2 control samples in 1 plate and 54 colonies from the A4 well in the other plate) with 2 primer sets.
We are going to have a heater-shaker module available but not a thermocycler.
1 of the source plates will have a map of identities, but the other will not have it.
We will use a computer with a Windows 10 system
Prepare variable file
Excel temple filled and saved with the name VariablesPCR.xlsx
Start run
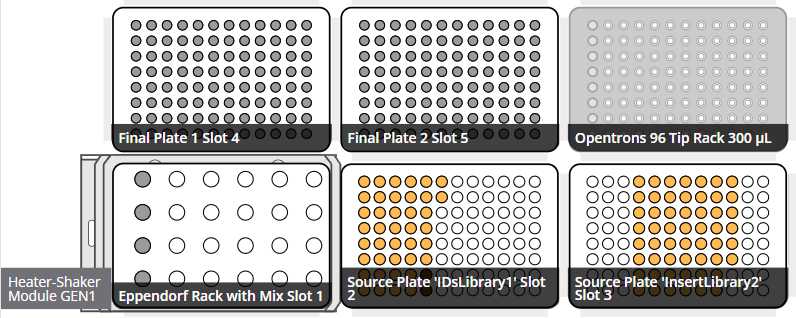
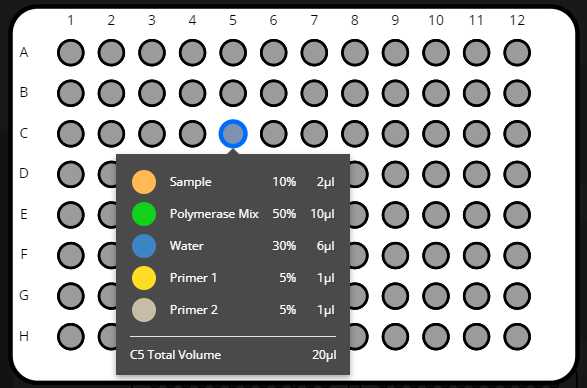
Retrieve labwares from the OT
Retrieve the final maps that are going to be in the file. In this case, they will be called example-mapPCR-example.xlsx (name that is stated in the variable file in the variable Final Map Name )
Upload custom labware to app
We are using a custom labware called vwrblueprintdepth105_96_wellplate_390ul and 3dprinted_opentrons_shaker_1.5mleppendorf that has been created with the labware creator that opentrons offer (https://labware.opentrons.com/create/)
vwrblueprintdepth105_96_wellplate_390ul.json
3dprinted_opentrons_shaker_1.5mleppendorf.json
Upload it to the labware and make sure it is loaded in the app
Because we are using version 1.0.0 of the script in this example, we will transfer the directory of the labwares as well (here we have attached a zip, but it is the folder the one that must be transferred, not the zip)
vwrblueprintdepth105_96_wellplate_390ul.zip
3dprinted_opentrons_shaker_1.5mleppendorf.zip
#Transferring the used custom labware to OT (Linux)
scp -i [ot_key] -r vwrblueprintdepth105_96_wellplate_390ul root@[IP_OT]:/data/labware/v2/custom_definitions/custom_beta
#Transferring the used custom labware to OT (Linux)
scp -i [ot_key] -r 3dprinted_opentrons_shaker_1.5mleppendorf root@[IP_OT]:/data/labware/v2/custom_definitions/custom_beta
Run the protocol in the robot that we have transferred the Excel file
Example-ScriptPCR.py Example-ScriptPCR.py -> Start setup -> Select robot in which we are going to run the protocol
If we do not have any errors, the output should look similar to the following pictures
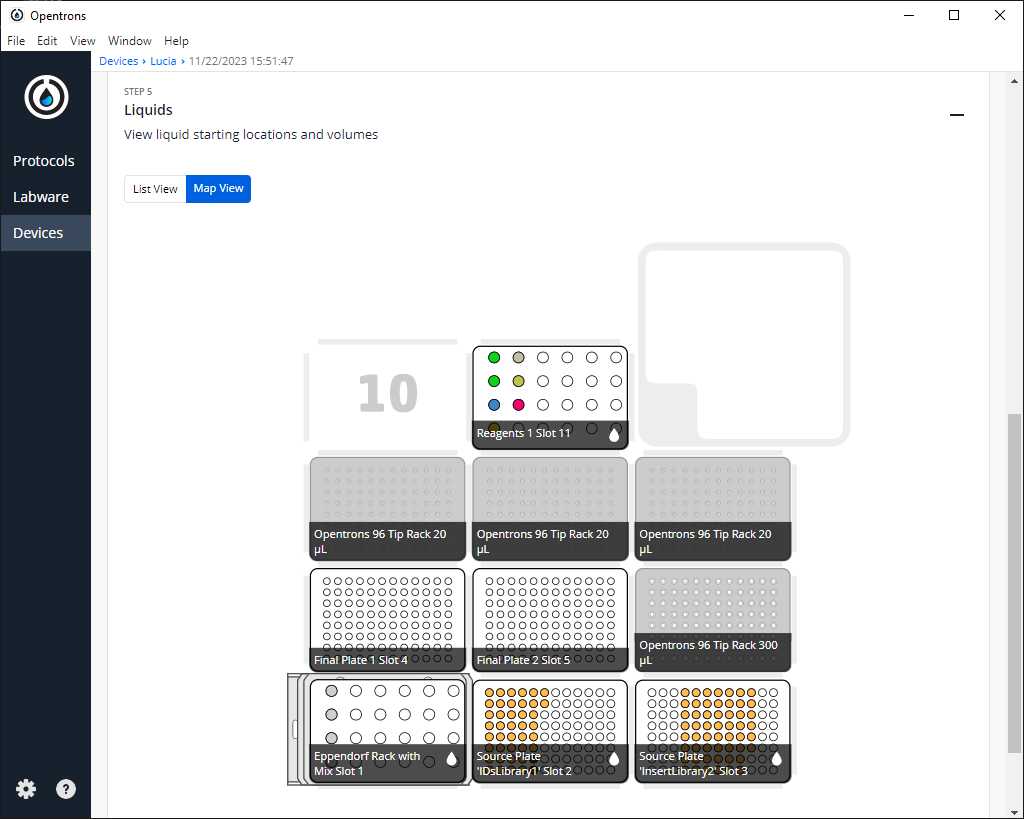
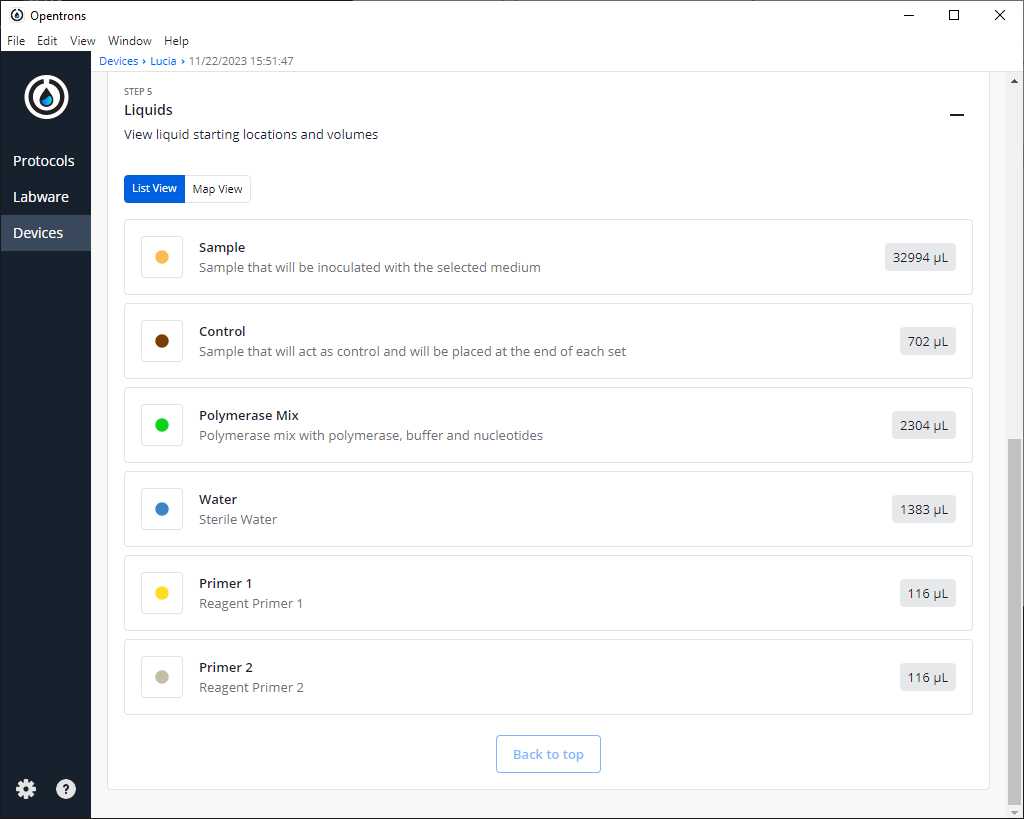
Turn the HEPA filter module
Clean the platform of the robot that we are going to perform the protocol
Prepare all reagents and labware in the places the App is showing and take into account the notes in step