Novel tank test in zebrafish
Ivoneide Maria Menezes Barra, Bruna Patrícia D.c., Leonardo Miranda Feitosa, Wilker Leite do Nascimento, Monica Gomes Lima-Maximino, Caio Maximino
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
The novel tank test (NTT) is widely used in zebrafish to either screen anxiolytic-like or anxiogenic-like effects of compounds or as a behavioral bioassay to understand the neurobiology of defensive (anxiety-like) behavior. The test exploits zebrafish's natural preference for the bottom portions of a novel tank, a consequence of its biotic and abiotic environment in the wild. Advantages of the NTT include its ease of implementation as a fast assay, as well as its sensitivity to anxiolytic and anxiogenic compounds. The main endpoint is the time the animal spends in the bottom third of the tank; secondary endpoints include erratic swimming, freezing, homebase, and swimming speed. The protocol is suitable for adult zebrafish, but has also been applied to other small teleost fish, including the jundiá Rhamdia quelen and the medaka Oryzias latipes .
Steps
Preparation
Separate materials for the experiment.
Tank variations have been reported in the literature. In our lab, we use simple rectangular glass tanks with transparent walls (25 x 24 x Y cm [width X height X length]), filled with 5 L of system water. Other groups have used trapezoidal tanks.
The back and sides of the tank should be covered in white adhesive paper to occlude the animal's vision and improve contrast for automated tracking.
Position the tank above a bench with vibration minimization.

Position the tank in a well-lit bench. Lighting the tank from the top increases bottom-dwelling, and lighting it from below should be avoided. Measure light levels with a light meter. Ideally, light levels should fall between 250 and 300 lux, with a coefficient of variation of less than 5% between subjects.
Equipment
| Value | Label |
|---|---|
| Light Meter LI-250A | NAME |
| Light Meter | TYPE |
| Li-Cor Biosciences | BRAND |
| . | SKU |
| https://www.licor.com/bio | LINK |
| Part Number: LI-250A | SPECIFICATIONS |
Position a video camera in front of the tank, at a distance of 40 cm of the tank. We currently use mobile phone video cameras, given the high quality of the videos which are produced by these cameras.
Using a computer connected to a loudspeaker, initiate playback of white noise. Many different online websites are now able to generate and playback white noise (e.g., https://noises.online/). Measure noise with a decibel meter. Sound levels should not be above 60 dB as measured above the tank.
Drug treatment
Anesthesize animals in ice-cold water.
Add ice to a beaker filled with at least 1 L system L. Measure temperature so that it falls between 10°C to 12°C
Add a tea strainer above the ice layer, where individual animals will be placed. The use of the strainer allows cooling the water while avoiding direct contact of the fish body with the ice.
Transfer animals individually to the strainer. Monitor fish behavior: At the start of the anesthetical plane, the fish typically will spread its pectoral fins horizontally, gasp, and have rapid operculum movements. Slowing of opercular movements and cessation of gasping is a sign that the surgical plane is approaching. When the animal no longer reacts to being handled, it is ready to for injection.
Perform injection
Quickly transfer the animal to the surgical table, soaked in cold water, and position the animal with the abdomen up and the gills covered by the sponge.
Carefully insert the needle of the syringe in the midline between pelvic fins, with the needle pointing slightly cranially. The needle should be inserted closer to the pelvic girdle than to the anus.
Inject the appropriate volume in the animal. For adult animals (between 25 and 40 mm standard length), a volume of 5 µL is sufficient.
Quickly transfer the animal to system water in room temperature, monitoring behavior until full recovery.
Behavioral assay
Start filming in the camera.
Quickly and gently transfer the animal to the tank, allowing the animal to freely swim for 6 minutes, recording the test all along.
Stop the camera and remove the animal.
Tracking and behavioral scoring
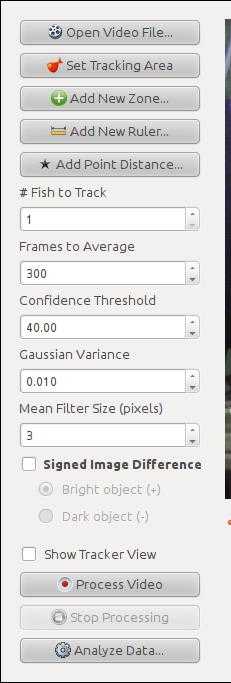
Transfer video files to a computer running Windows or Linux to post-processing using TheRealFishTracker
Software
| Value | Label |
|---|---|
| TheRealFishTracker | NAME |
| Windows | OS_NAME |
| 32 bits | OS_VERSION |
| James McCrae | DEVELOPER |
| https://www.dgp.toronto.edu/~mccrae/projects/FishTracker/ | LINK |
| 0.4.0 | VERSION |
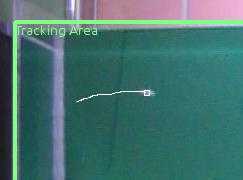
Click "Set Tracking Area" to specify a rectangular region into the video that covers the tank. Make sure the whole region the fish can move is bound by the rectangle.
Click "Add New Zone" to specify a rectangular region covering the bottom third of the tank. This zone will be used to quantify geotaxis, the main endpoint of this test.
Click "Add New Ruler". Trace a line following the width of the tank, name it "Width", and set its length as "25" (or the actual width of your tank).
Click "Add New Ruler". Trace a line following the height of the tank, name it "Height", and set its length as "24" (or the actual height of your tank).
To start the tracker, click "Process Video"
If the software fails to detect the fish or to trace its swimming path during tracking, it may be necessary to adjust tracking parameters. If the fish is too small and is not being detected, try decreasing "Mean Filter Size". If the quality of the recording is low, try decreasing the "Confidence Threshold" to 20.
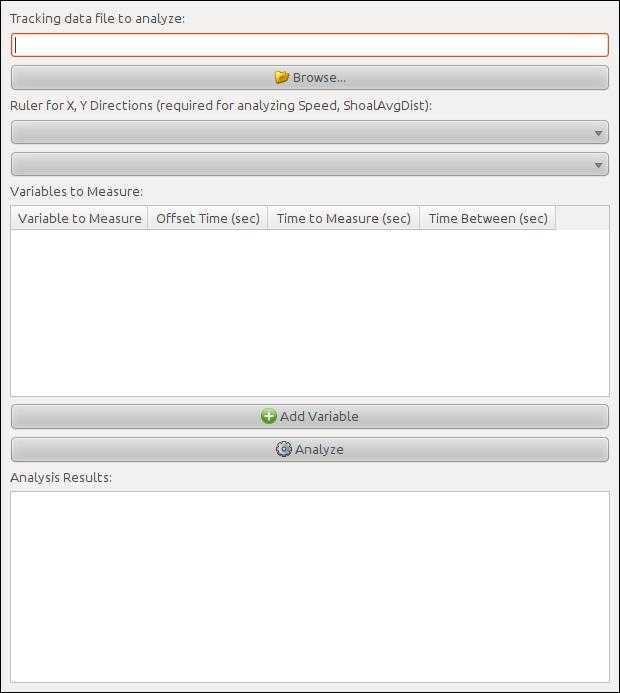
Extract data from the processed file
Click "Browse" to specify the tracking data file. The filename defaults to the name of the video appended with the extension .data.txt.
Specify the rulers in the dropdown menus. The rulers that will be used were those that were configured in Step 11.
Click on "Add variable" to add the following endoints:
- Zone1-bottom: To analyze the time spent in the bottom third of the tank
- AbsoluteTurnAngle: To analyze erratic swimming
- Speed: To analyze swimming speed
Click on "Analyze" to produce a plaintext analysis of variables per second in the bottom window ("Analysis Results"). Click on this window, press CTRL + A to mark all data and CTRL + C to copy it. Paste the content on a Notepad file, and save it with the .csv extension.
Open the newly-created .csv file on a spreadsheet software, such as Microsoft Excel or LibreOffice Calc.
The file will consist of lines and columns with each variable titled with the endpoint name, with mean and variance values separated by second. To transform variables into session totals, you will need to use formulas, as follows:
- For bottom-dwelling/geotaxis, sum all values in the "mean" line with the "SUM" function
- For erratic swimming and speed, take the average of all values in the "mean" line with the "AVERAGE" function.
To extract data for freezing, create a line below the "mean" line, and add a formula:
#Command to extract data for freezing from swimming speed. (LibreOffice Calc)
=IF([cell above the command] < 0.5;1;0)
#substitute the name of the cell
```You will need to copy this formula throughout the whole line in order to generate "1s" and "0s" for every second of the tracking period.
After that last step, sum all generated values using the "SUM" function.
Copy and paste generated values in another spreadsheet for statistical analysis.