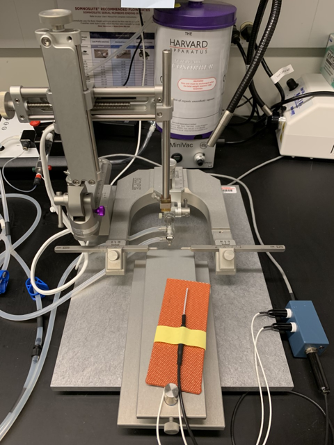
Mouse Stereotaxic Surgeries for Intracranial Viral Injection
taylor.panczyk
Abstract
This procedure allows to inject a small volume of solution (in our case, either a suspension of genetically modified viruses, that will infect neurons and will induce the expression of desired proteins, often genetically-encoded probes; or a suspension of a chemical neuronal marker, “fluorogold”, that will be taken up that neurons with axonal projections in the area of injection) in a specific region of the brain.
An anesthetized mouse is placed on the stereotaxic apparatus, where its head is immobilized and positioned so that once the skull is exposed, specific anatomical landmarks (usually, the bone sutures) can be identified and used to calculate the relative position of different brain areas expressed as x/y/z coordinates. small hole can be drilled in correspondence of the desired x/y coordinates and the injection pipette can then be lowered to desired z coordinate, where the solution is slowly released.
After suturing the mouse and waiting an appropriate time for recovery and expression of the protein of interest, the mouse can be sacrificed and used for experiments.
Steps
Surgical Set
Prepare a clean empty mouse cage on a heating pad and a clean mouse cage with gel food for post-op care
Set up sterile working area including stereotaxic frame
Weigh mouse
Anesthetize mouse in induction chamber (recommended: 2.5% isoflurane, 200ml/min flow rate)
Once the mouse is deeply anesthetized (~0h 5m 0s), stop anesthesia and move the mouse to the stereotaxic frame over the heating pad with the temperature probe and secure the mouse mouth on the nose cone.
Restart anesthesia (directed towards the nose cone)
The heating pad settings should be adjusted so that the temperature probe placed under the mouse should read a body temperature between 33°C - 37°C
Apply ophthalmic ointment over eyes
Inject appropriate volume (based on mouse weight and desired dosage) of analgesic; an appropriate amount of saline can also be injected to prevent dehydration during the procedure
Carefully insert and secure the ear-bars. The position of the mouse head will be verified and adjusted once the skull is exposed, but it is recommended to make sure that the head is not visibly tilted
Clean the area of the incision with the povidone-iodine swab followed by the ethanol swab
Repeat Step 11, 3 times
It is preferred to apply line-block anesthetic (0.15% bupivacaine) under the skull skin before starting the procedure rather than applying EMLA cream on the sutured skin at the end of the surgery
Surgical Procedure
With the fine scissor, expose the skull by making an anterior-posterior incision
Visually identify bregma and lambda
Insert a glass pipette (a small volume of non-toxic food dye can be used to help marking the relevant spots) on the stereotaxic arm holder and lower it onto the skull
Mark bregma by gently touching the intersection of the coronal/sagittal sutures with the pipette tip, and zero the coordinates on the reader
Move to lambda (intersection of lambdoid and sagittal sutures) and measure its position relative to bregma
Minimize the deviation of dorso/ventral (D/V) and medio/lateral (M/L) distance between lambda and bregma by adjusting the position of the head
Re-zero the coordinates at bregma and repeat bregma/lambda measurements until satisfactory
Once the head is in the correct position, it is possible to identify the desired injection spot
It is recommended to use the measured anterior/posterior (A/P) distance between bregma and lambda to calculate an adjustment factor for the final coordinates: the measured B-L distance will be divided by the reference distance of 4.21. For an adult mouse, the obtained value (“adjustment ratio”) should be close to 1, and in this case no coordinates adjustment is required (but still optional). For smaller mice, the reference coordinates should be multiplied by the calculated adjustment ratio to obtain the final coordinates for the specific mouse
Move the pipette to the spot indicated by the adjusted A/P and M/L coordinates and mark it
Whether performing uni-lateral or bi-lateral injections, it is recommended to mark the spots on both sides of the skull, and to measure their relative dorso-ventral position. Their relative D/V deviation should be minimized by adjusting the position of the head
Once the desired spot has been marked, the marker pipette can be removed, and a hole is drilled in the skull at the indicated position
Blood and debris are cleaned with sterile saline and sterile cotton swabs
Insert micropipette with volumetric references in the holder and connect it to a syringe to apply positive/negative pressure
Draw up desired volume of viral solution in the syringe by applying negative pressure
Lower pipette loaded with the viral solution into the hole until the tip touches the dura. Zero the dorso-ventral coordinate
Gradually lower the pipette tip into the brain until the desired dorso-ventral coordinate is reached
Slowly inject the desired volume of viral solution (recommended: ~150nl/min) by gently and gradually applying positive pressure
Release pressure and leave the pipette in position for ~5-10 min so that the viral solution can spread and be absorbed by the tissue
Slowly retract viral injection pipette and discard it in an appropriate waste collection bin
Suture Skin
Optional: Repeat saline injection to prevent dehydration
Post-Surgery
Remove animal from stereotaxic frame and place it in the clean, empty cage on heating pad until deambulatory (~0h 10m 0s - 0h 15m 0s
Once awake and deambulatory, mouse can be moved to the clean cage with gel food, also on heating pad
24h 0m 0s after surgery, a second dose of Metacam is administered and antibiotic ointment is applied on the sutured skin
The health status of the mouse is monitored over the following days. If needed, additional doses of Metacam or saline can be administered
Mouse is normally kept in a cage on heating pad for at least 4 days and is then moved to standard housing
Mice are sacrificed for experiments at least 10 days after surgeries