Measuring protein concentration using the Merck Millipore Direct Detect Spectrometer
ronan o'cualain
Millipore Direct Detect
Measuring protein concentration
S-Trap buffer
protein estimation
spectrometer
quantitation
Bradford
BCA
Abstract
This protocol details the procedure of measuring protein concentration using the Millipore Direct Detect spectrometer.
Before start
Initial preparation
Before you begin:
Identify the following equipment that you will use:
- 10uL pipette and pipette tips
- Millipore Direct detect sample cards (DDAC00010-GR)
- Millipore Direct detect machine (C134681)
Attachments
Steps
Loading samples on card:
Label the bottom membrane on the card for blank measurement.
Label a clean 0.75mL Eppendorf tube with “pool”. To it, add 2µL of each of your samples. When complete, vortex mix briefly. A pooled sample should be representative of the batch you wish to measure, such as a set of replicates.
Pipette 2µL of blank into center of the membrane designated as the blank position.
Pipette 2µL of your pooled sample to be analyzed into center of the three remaining spots.
Software and measurement:
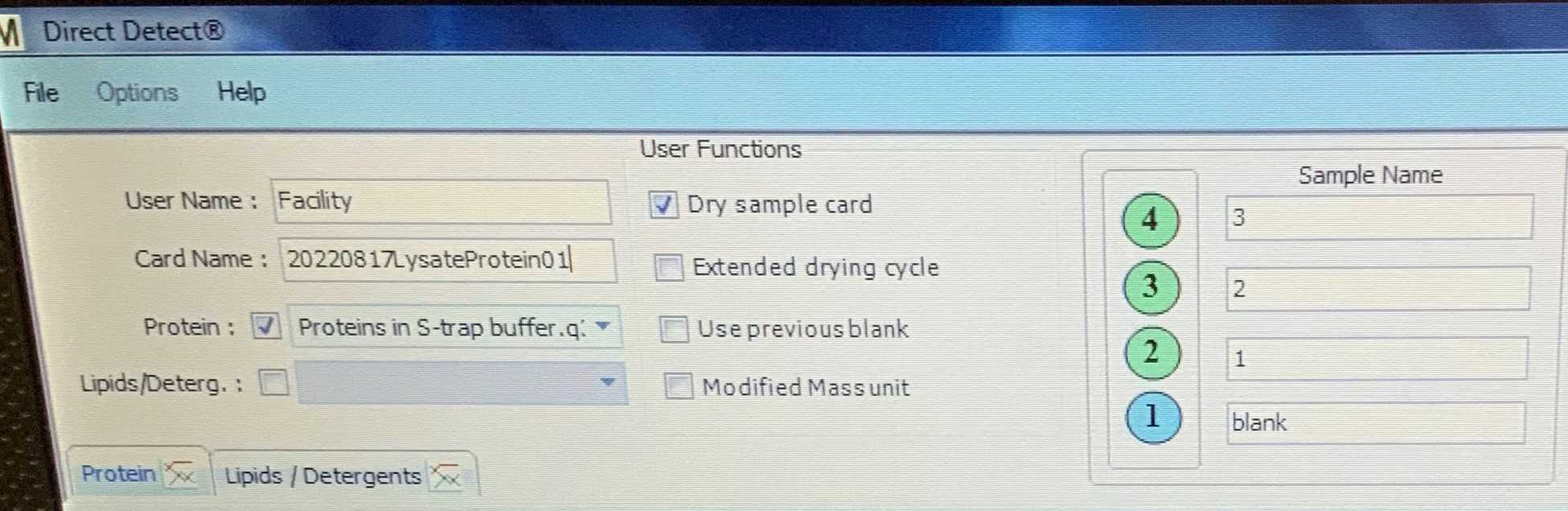
In the software, complete the fields as follows:
a. User name should be preset (Facility).
b. Card name – today’s date, followed by your initials and the sample number.
c. Protein – use the drop down menu to select “proteins in S-Trap buffer.q3”.
d. Ensure that the box marked “ dry sample card ” is ticked.
e. Do not tick the boxes marked “lipid”, “extended drying cycle”, “use previous blank”, or “modified mass unit”.
f. Give the 4 card positions a name, the position marked 1 in blue is the blank , while the other three should be in green, these are your three replicate measurements.
Click on the measure card button.
The sample concentrations will appear on the screen, along with the statistical analysis and spectrum plot.
After all four positions on the card have been read, the instrument will sound a tone. The card will rise to the initial insertion position. Remove the card and dispose of it.