Measuring fungal anti-E. coli activity using the zone of inhibition (ZOI) assay
Siouxsie Wiles, Shara Van De Pas
Abstract
In this protocol, we describe our use of the zone of inhibition assay to assess the anti- E. coli activity of fungi grown on different media, including Czapek Solution Agar (CSA), Czapek Yeast Extract Agar (CYA), Malt Extract Agar (MEA), Oatmeal Agar (OA), Potato Dextrose Agar (PDA), Rice Extract Agar (REA), and Water Agar (WA).
Before start
Steps
Initial culturing of fungus from stocks
Subculture fungus onto a Potato Dextrose Agar (PDA) plate from either frozen stocks or an existing culture.
Seal the plate with parafilm and store it in a plastic box at room temperature. Allow fungus to reach at least 50% radial growth before subculturing onto respective media plates.
Culturing of fungus onto different media
Using a sterile scalpel plate, cut a small section (0.5cm) from the growing edge of the mycelium and inoculate this segment onto fresh media. In our experiments, we routinely subculture fungi onto a selection of different media.
Monitor radial growth of the fungus and start the ZOI assay when it reaches 20, 50, and 100% growth.
Setting up E. coli 25922 lux and ZOI plates
Inoculate 10 mL of Mueller Hinton II broth in a 15 mL tube with E. coli and grow overnight at 37 degrees C with shaking at 200 rpm. We use a bioluminescent derivative of the antibiotic-testing E. coli ATCC 25922 strain designated 25922 lux.
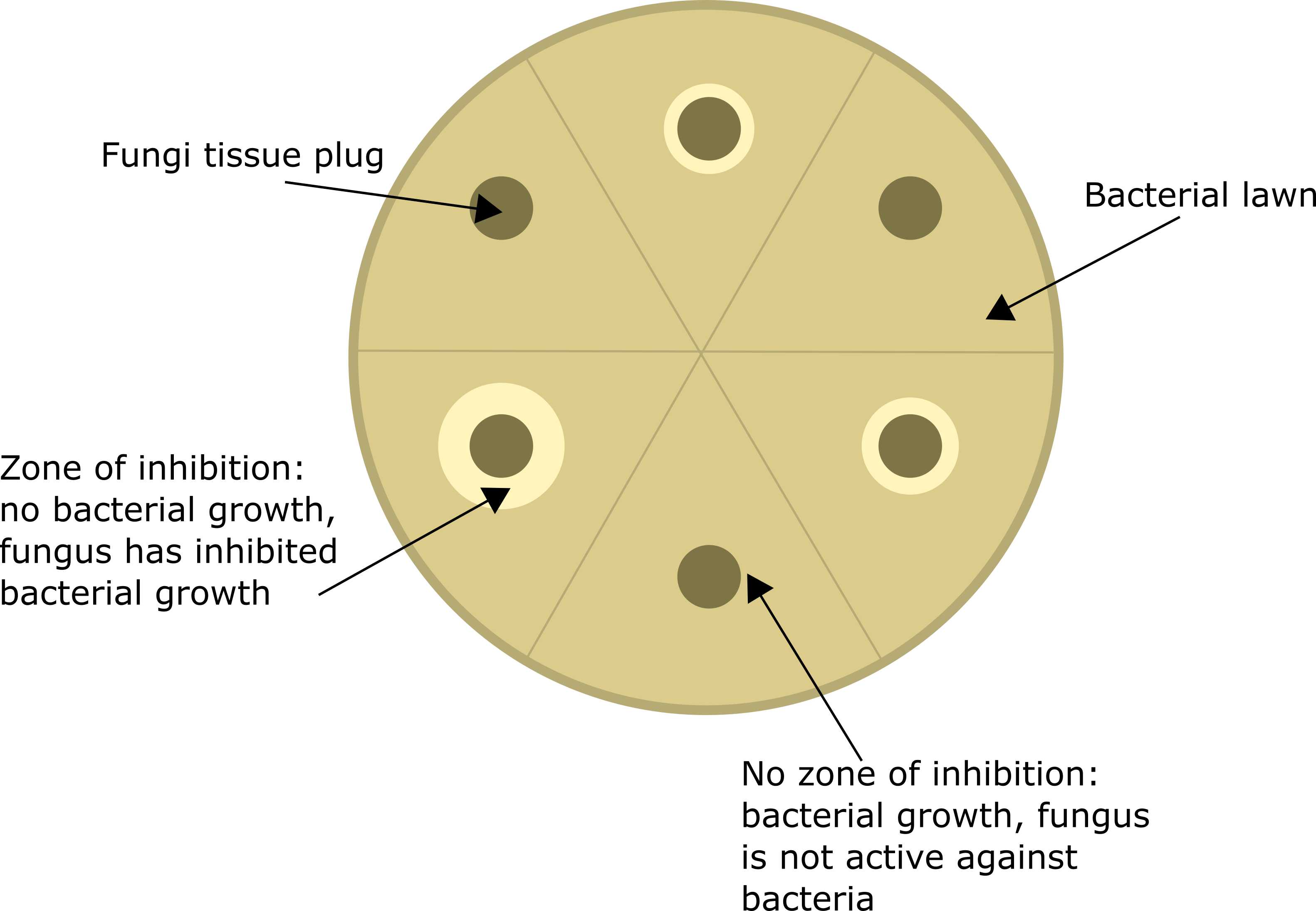
In these assays, we perform the ZOI on the same media the fungus has been growing on after confirming that E. coli 25922 lux growth is not impacted. Prepare the necessary number of plates of each media depending on how many fungal isolates, and biological and technical replicates of each isolate, you need to test. Once dry, section the back of each plate into six equal segments. Label each with the fungal isolate name/number and the date the fungus was cultured (age).
Measure the optical density of the overnight bacterial culture at 600nm (OD600). To do this dilute overnight cultures 1:10 in a 1.5mL cuvette with Mueller Hinton Broth (MHB) ( 720µL broth + 80µL bacteria). Dilute the bacterial culture with MHB to give a final OD600 of 0.01 which is equivalent of ~106 bacteria per mL.
Add bacteria to each Petri dish. This can be done by pipetting 50ul of diluted overnight bacterial culture and spreading it over the plate using an L-shaped spreader. Alternatively, dip a sterile cotton swab into the diluted bacterial solution and gently streak the agar plate half a section at a time rotating 45 degrees each time to ensure an even lawn of bacteria.
Allow plates to dry before applying fungal plugs.
Adding fungal plugs to ZOI plates
Use a 6mm punch biopsy to remove mycelium from the growing edge of the fungus. Place the plug fungus-side down on your labelled plate. Repeat for all biological/technical replicates.
Use a sterile punch biopsy when changing fungi to avoid cross-contamination.
Also prepare a no-fungi control by adding an agar-only plug to the bacterial lawn. Ensure the agar is the same as that the fungus was grown on.
Incubate the plates upside down at 37°C in a standing incubator overnight.