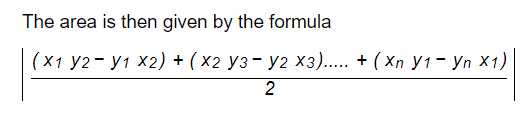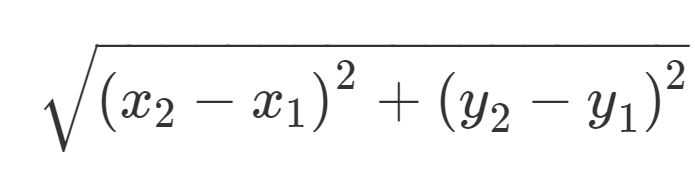Measuring Coral Skeletal Architecture Using Image Analysis
Rowan Mclachlan, Ashruti Patel, Ashruti Patel, Andrea G Grottoli
morphometrics
morphometry
micromorphology
microstructure
corallite
septa
theca
columella
skeleton
aragonite
microcorallite morphology
coral
coral reef
Abstract
Coral morphology is influenced by genetics, the environment, or the interaction of both, and thus is highly variable. This protocol outlines a non-destructive and relatively simple method for measuring Scleractinian coral sub-corallite skeletal structures (such as the septa length, theca thickness, and corallite diameter, etc.) using digital images produced as a result of digital microscopy or from scanning electron microscopy. This method uses X and Y coordinates of points placed onto photomicrographs to automatically calculate the length and/or diameter of a variety of sub-corallite skeletal structures in the Scleractinian coral Porites lobata. However, this protocol can be easily adapted for other coral species - the only difference may be the specific skeletal structures that are measured (for example, not all coral species have a pronounced columella or pali, or even circular corallites). This protocol is adapted from the methods described in Forsman et al. (2015) & Tisthammer et al. (2018).
There are 4 steps to this protocol:
-
Removal of Organic Tissue from Coral Skeletons
-
Imaging of Coral Skeletons
-
Photomicrograph Image Analysis
-
Calculation of Corallite Microstructure Size
This protocol was written by Dr. Rowan McLachlan and was reviewed by Ashruti Patel and Dr. Andréa Grottoli.
Acknowledgments
Leica DMS 1000 and Scanning Electron Microscopy photomicrographs used in this protocol were acquired at the Subsurface Energy Materials Characterization and Analysis Laboratory (SEMCAL), School of Earth Sciences at The Ohio State University, Ohio, USA. I would like to thank Dr. Julie Sheets, Dr. Sue Welch, and Dr. David Cole for training me on the use of these instruments.
Steps
Removal of Organic Tissue from Coral Skeletons
Remove coral fragments from the freezer (if applicable) and place them in individual 500 ml plastic beakers filled with deionized water. Soak coral fragments for 30 minutes at room temperature to remove excess salt and prevent NaCl precipitation.
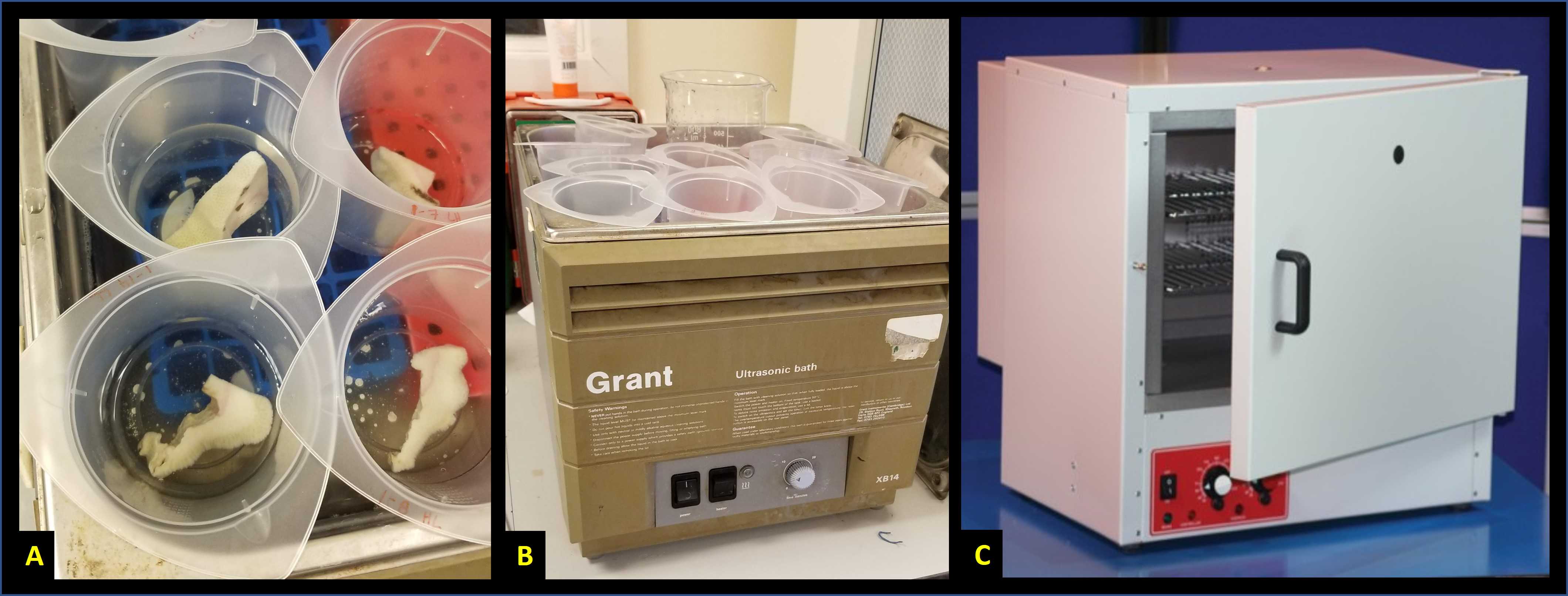
Using plastic forceps, transfer coral fragments to new 500 ml beakers filled with ~ 200 ml of 3% sodium hypochlorite bleach (Fig 1A). Take care not to damage fragile corallite micro-skeleton. Soak for 1 hour at room temperature.
Replace liquid contents of the beakers with fresh bleach solution and arrange beakers in a sonicator bath (Fig. 1B). Sonicate for approximately 30 minutes at room temperature.
After sonication, visually inspect skeletons. If the coral skeletons are completely white and devoid of tissue, proceed to the next step (Step 5). However, if tissue is still visible (i.e., the coral surface is light brown or green) then sonicate skeletons in fresh bleach solution for an additional 30 minutes (or longer) until all tissue disappears.
Drain and dispose of bleach, transfer coral skeletons into individual pre-labeled aluminum weighing pans, and dry overnight at 60 °C in a drying oven (Fig. 1C).
Imaging of Coral Skeletons
The photomicrographs used in this protocol were produced using a Leica DMS1000 Microscope. For detailed instructions on how to use this instrument, please refer to the User Manual.
Multiple photomicrographs should be taken per individual coral skeleton at a variety of angles and magnifications (Fig. 2). First, take lower magnification images in order to visualize the gross morphology of the skeleton (Fig. 2 A). Next, take medium-magnification images from above (i.e., dorsal view) in order to visualize individual corallites (Fig. 2 B). Finally, take multiple high-magnification images to visualize sub-corallite structures (Fig. 2 C, D). Specific magnification levels vary and will depend on the size of coral fragments and the size of corallites. The angle of the skeleton relative to the microscope should be adjusted between high-magnification photomicrographs as later image-analysis measurements can only be conducted on corallites that were photographed perfectly perpendicular to the field-of-view rather than at an angle.

Photomicrograph Image Analysis
Download ImageJ. ImageJ can be downloaded for free from the NIH website: https://imagej.nih.gov/ij/download.html.
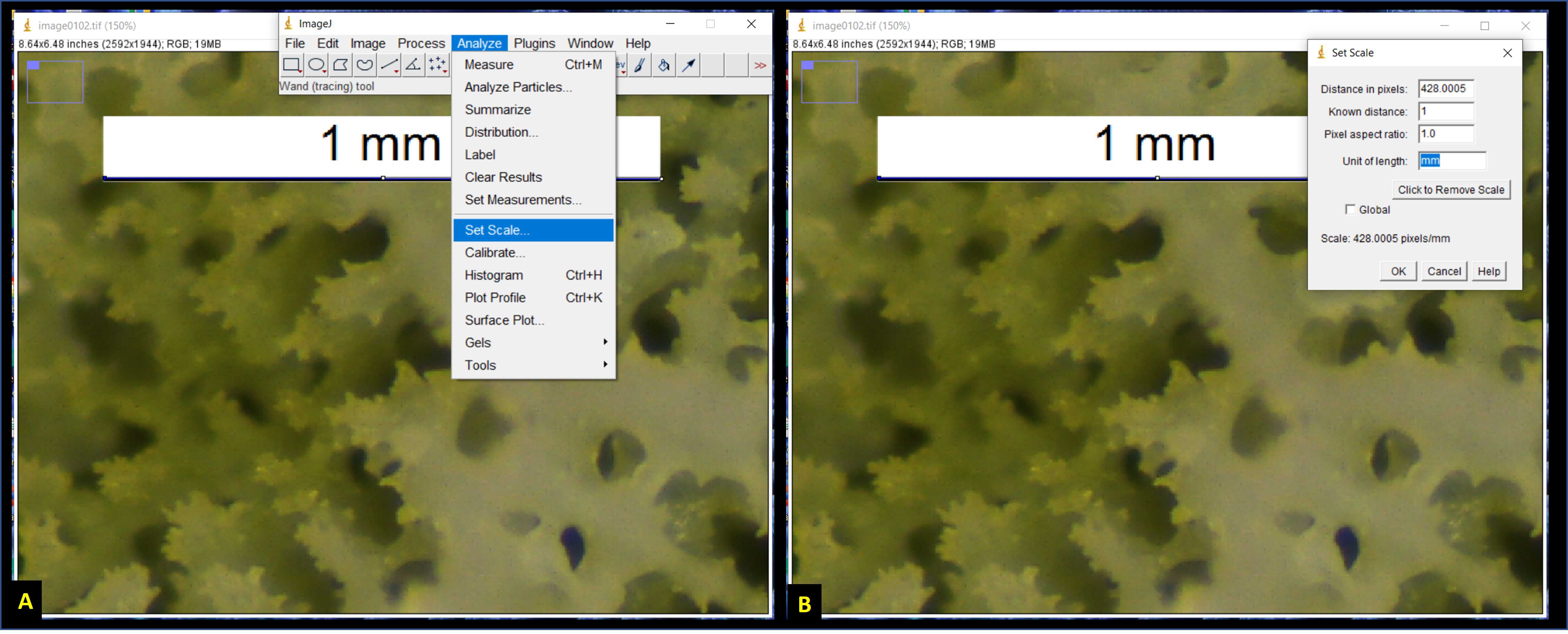
Set the scale. Using the hand tool and the zoom function (zoom shortcuts: Ctrl and + on PC keyboard, or left-click and scroll on PC mouse), zoom in to the upper left corner of the photomicrograph where the scale bar is located (Fig. 4A). Using the "Straight" tool (fifth button from the left), carefully select the entire 1 mm scale bar (Fig. 4A). Once the scalebar is selected, go to "Analyze" followed by "Set Scale" (Fig. 4A). In the Set Scale window, type "1" into the "Known Distance" field, and type "mm" into the "Unit of length" field (Fig. 4B).
After setting the scale, zoom out again (Fig. 5A), select an appropriately angled corallite, and then zoom in such that a single corallite fills the entire ImageJ window (Fig. 5B).
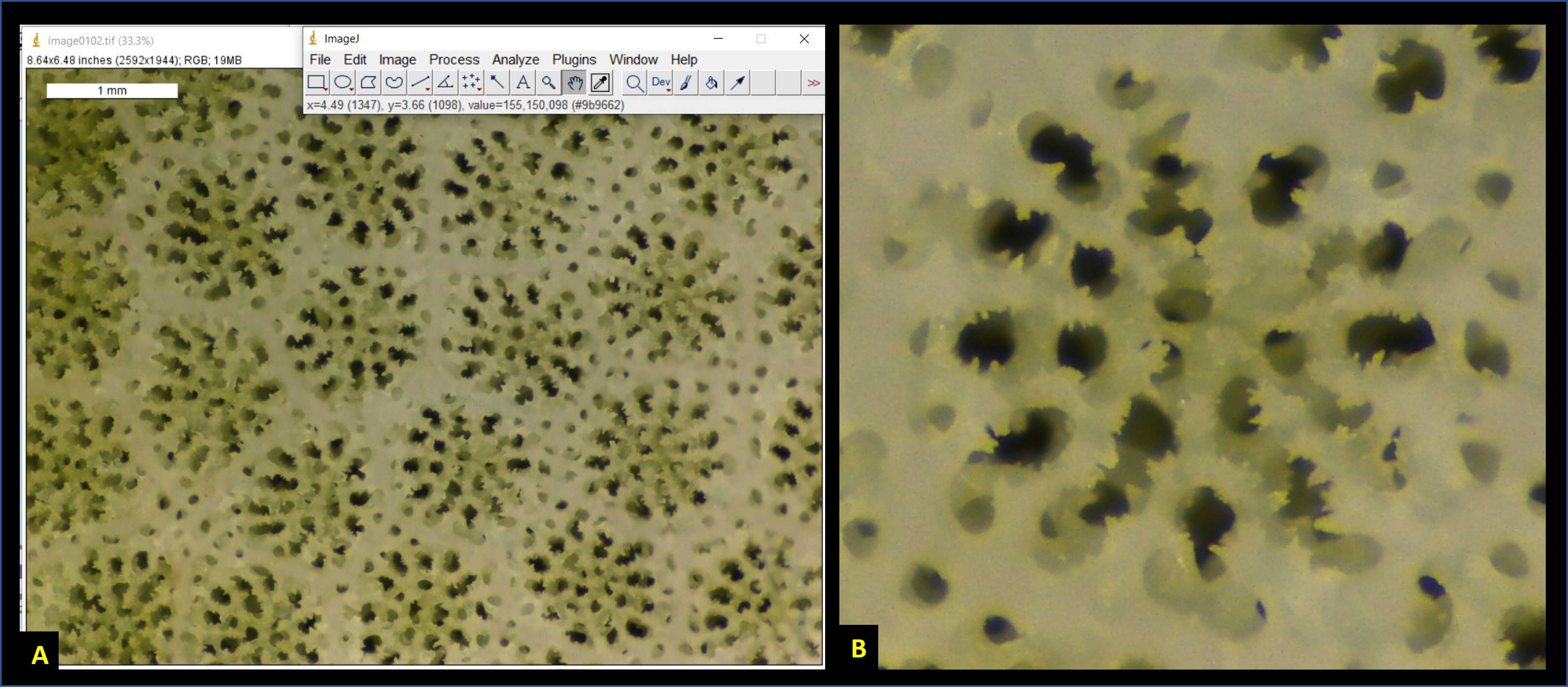
Make sure to only select corallites which are perpendicular to the field-of-view (Fig. 6). If the cupped-base of the corallite is at an angle relative to the field-of-view, then measurements made from images will not be accurate. You will notcie that in Fig. 6A, only the central corallite is appropriately angled, whereas those around it are all tilted.

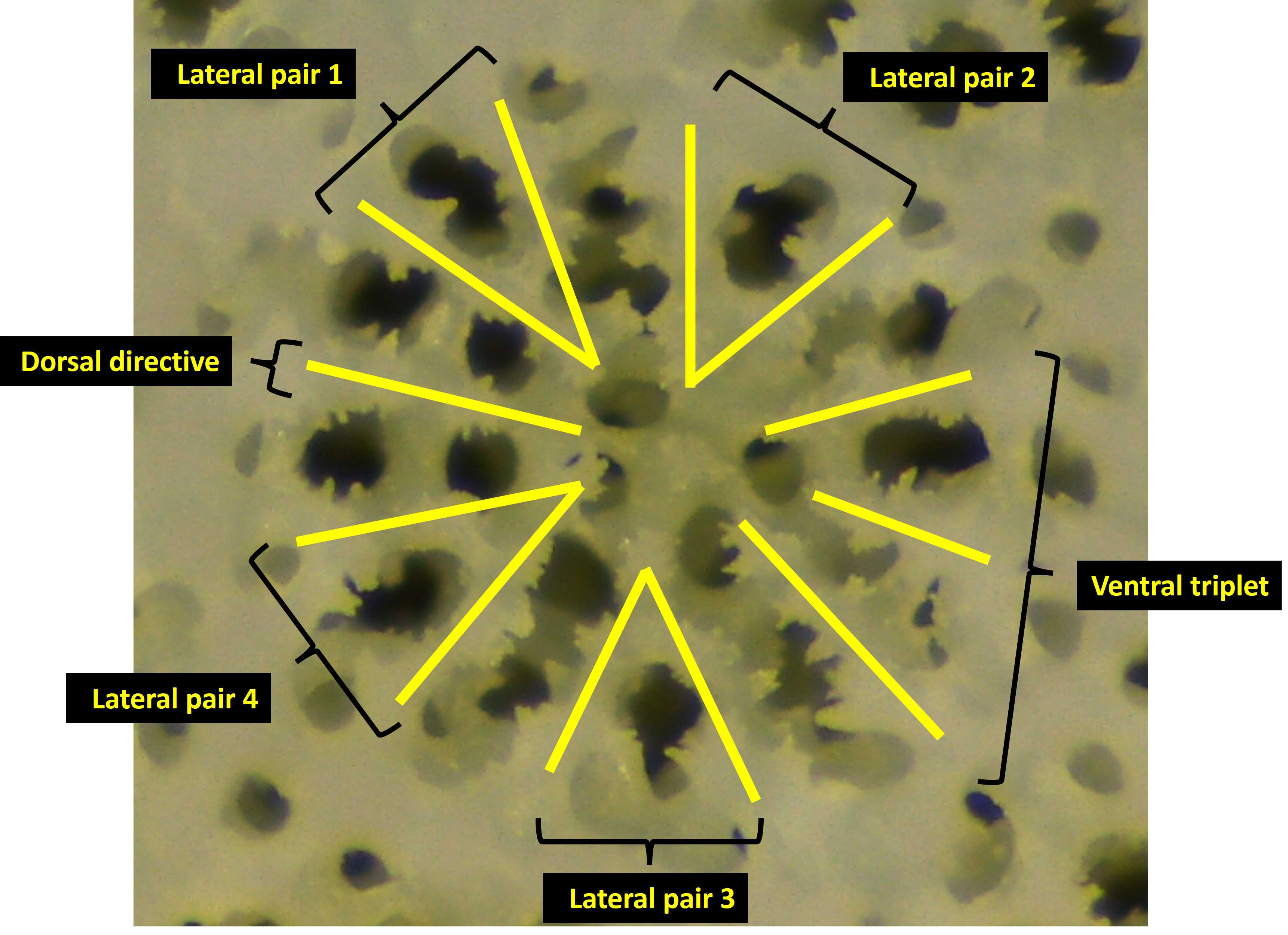
After selecting an appropriately-angled corallite, next, identify the dorsal directive septa (ubiquitous among Porites corals) (Fig. 7). Rotating clockwise from the dorsal directive are lateral pair 1, lateral pair 2, the ventral triplet, lateral pair 3, and finally, lateral pair 4.
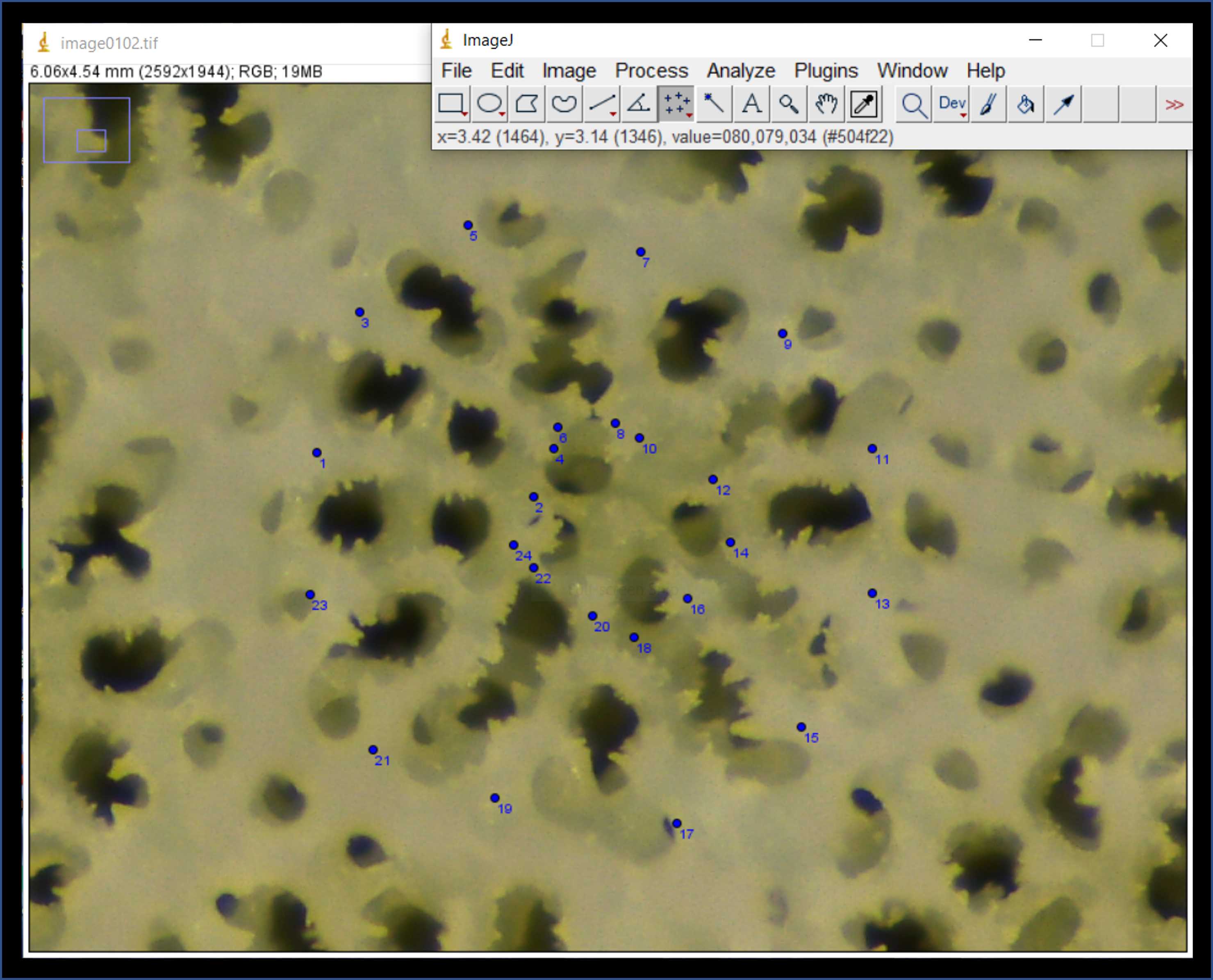
Select the "Multi-point" tool (seventh button from the left) (Fig. 8A). Place two points on top of the image. The first point should be placed at the outer end of the dorsal directive septa, and the second point on the inner end of the dorsal directive septa (Fig. 8B). These points will be used to calculate septal-length. Notice in Fig. 8 the small blue numbers next to each point (you may need to enlarge the image in order to see these numbers).
<img src="https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.bx5bpq2n/Picture10.jpg" alt="Fig. 8. A) Select the "multi-point" tool from the ImageJ toolbar, and B) place the first point (#1) at the outer end of the dorsal directive septa and place the second point (#2) at the inner end of the same septa." loading="lazy" title="Fig. 8. A) Select the "multi-point" tool from the ImageJ toolbar, and B) place the first point (#1) at the outer end of the dorsal directive septa and place the second point (#2) at the inner end of the same septa."/>
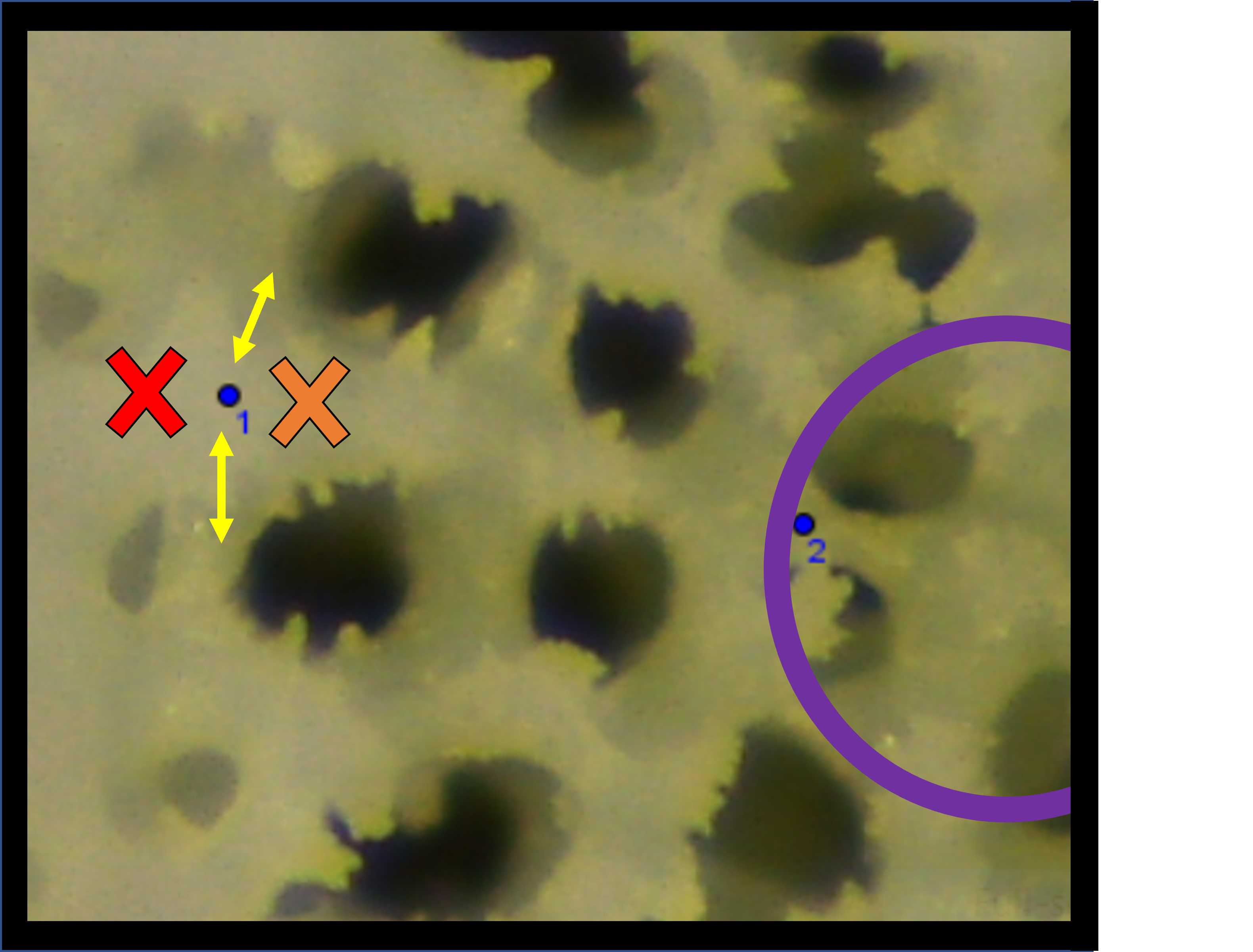
Take care when placing these septa end-point and try to be consistent within corallites, among corallites, and among different skeleton samples. One method we used to improve consistency was to use the negative space (black holes) as a guide (Fig. 9).
Notice in Fig. 9 how the blue point #1 is placed between the yellow arrows which align with the skeleton holes between septa. If the blue point had instead been placed where the red cross symbol is, it would have been too far out towards the theca wall. Conversely, if the blue point had instead been placed where the orange cross symbol is, it would have been too far inside the calyx area.
Likewise, for the placement of point #2, we used the ring of the fossa (purple circle in Fig. 9) as a guide and tried to place point 2 on, or as close to this ring as possible.
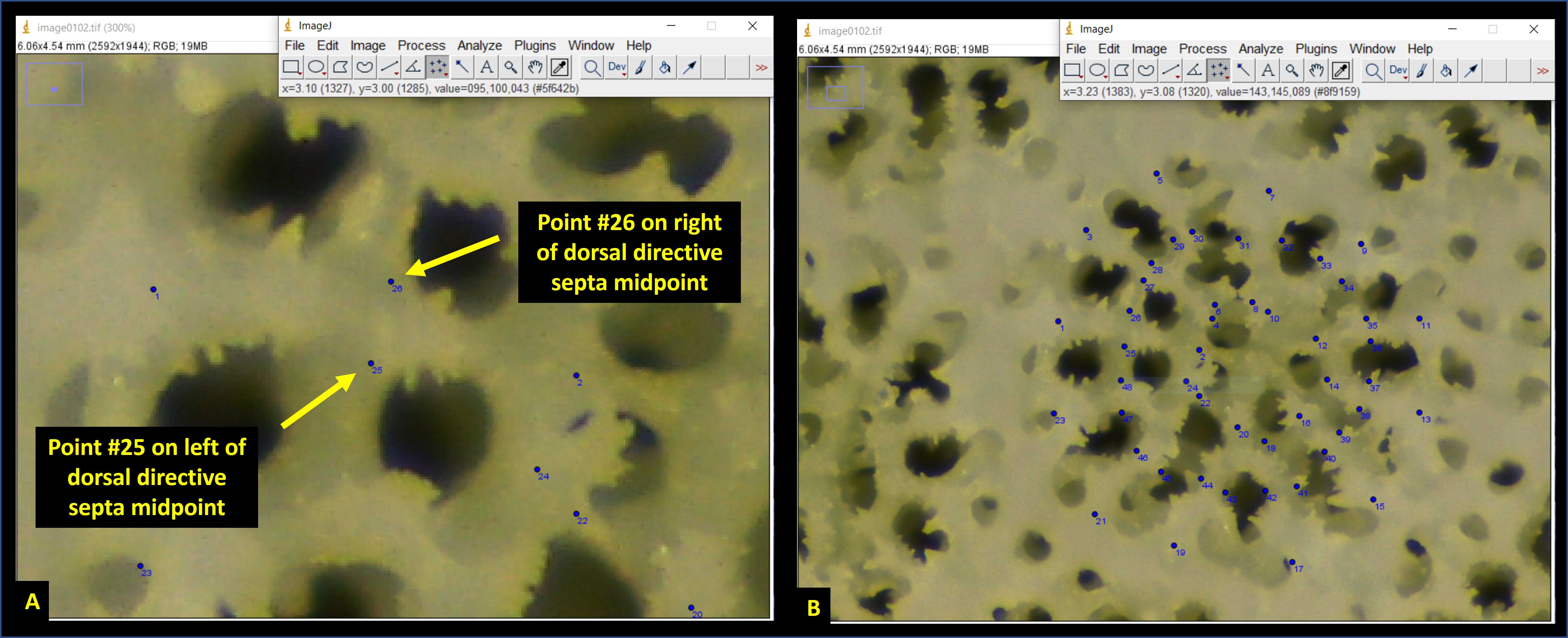
Next, place an additional 24 points on the photomicrograph on either side of each septa, located at the midpoint (i.e., halfway between the endpoints) (Fig. 11A). These points will be used to calculate septal-width. Proceed clockwise from the dorsal directive septa until points #25 through #48 have been placed (Fig. 11B).
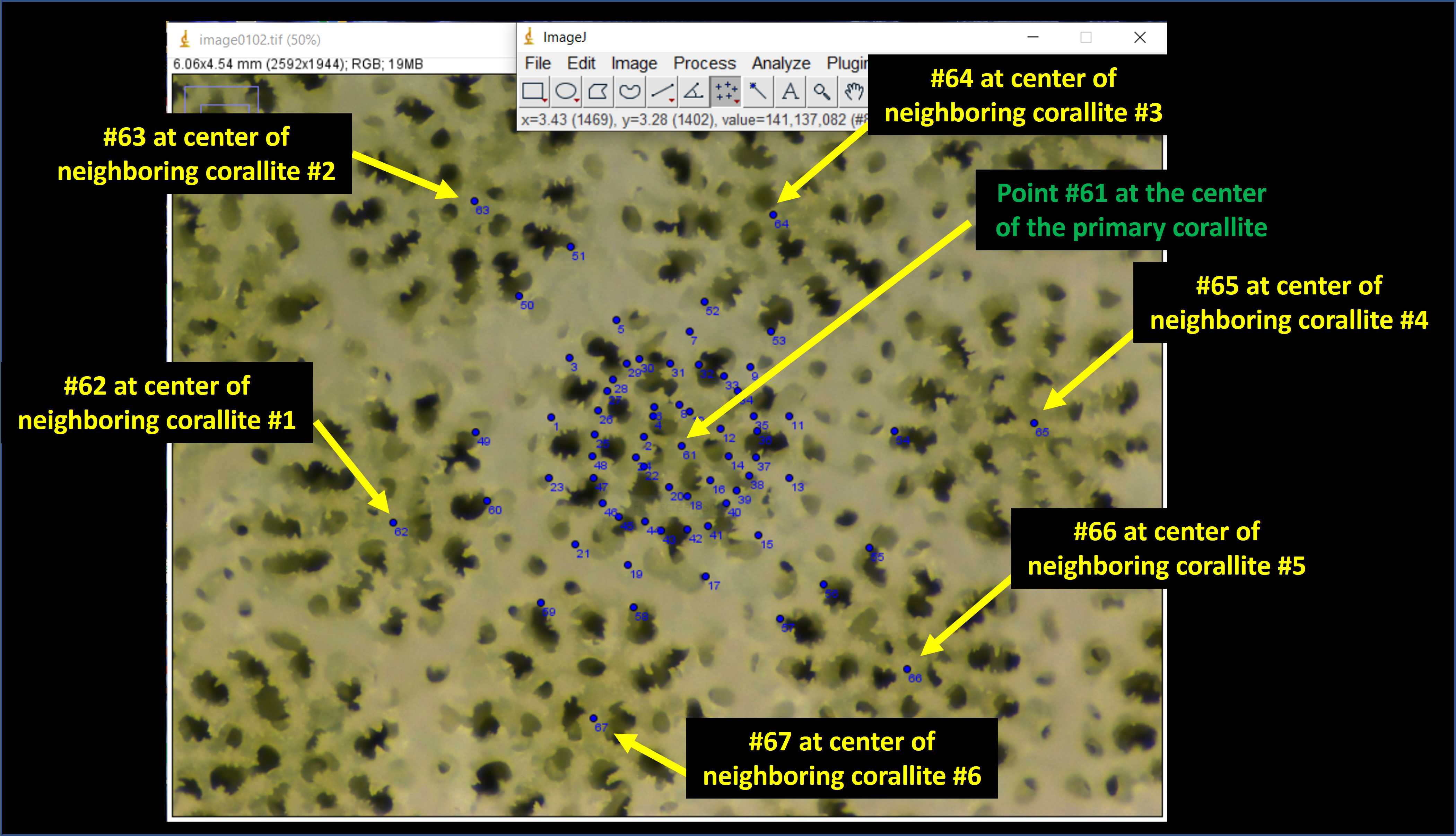
Next, place an additional 12 points on the photomicrograph opposite from the septa outer-edge points (Fig. 12A). These points should be placed on the inner edge of the theca walls of neighboring corallites (Fig. 12A). The distance between septa outer-edge points and these points will be used to calculate the thickness of the theca wall. Proceed clockwise from the dorsal directive septa until points #49 through #60 have been placed (Fig. 12B).

Next place point #61 at the center of the primary corallite (Fig. 13 green text). Following this, place one point in the center of all the neighboring corallites (only those which share a thecal wall with the primary corallite (Fig. 13 yellow text). The number of neighboring corallites will vary depending upon the individual (anywhere from 4–8 in my experience).
Next, navigate to the toolbar, press "Analyze" followed by "Measure" [keyboard shortcut is Ctrl + M] (Fig. 14A). This will bring up a Results window of the X and Y coordinates for all 67 points on the photomicrograph (Fig. 14B). Copy and paste these values into an excel spreadsheet.
<img src="https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.bx5bpq2n/Picture17.jpg" alt="Fig. 14. A) On the ImageJ toolbar, press "Analyze" followed by "Measure". B) Copy & Paste the X and Y coordinates for all 67 points from the Results window which appears." loading="lazy" title="Fig. 14. A) On the ImageJ toolbar, press "Analyze" followed by "Measure". B) Copy & Paste the X and Y coordinates for all 67 points from the Results window which appears."/>
Calculation of Corallite Microstructure Size
There are a variety of different measurements that can be made using this data set of X and Y coordinates. Table 1 summarizes which points can be used to calculate the length and width of various corallite microstructures. This is not an exhaustive list, however, and other measurements are possible with the placement of additional points (e.g., pali area, columella diameter, etc.) or from observations (e.g., number of radii, the shape of the ventral triplet, etc.). Please see Forsman et al. (2015) or Tisthammer et al. (2018) for such examples.
| A | B | C | D | E | F |
|---|---|---|---|---|---|
| Description | Points | Description | Points | Description | Points |
| Septa length 1 | 1:2 | Septa width 1 | 25:26 | Septa spacing 1 | 1:3 |
| Septa length 2 | 3:4 | Septa width 2 | 27:28 | Septa spacing 2 | 3:5 |
| Septa length 3 | 5:6 | Septa width 3 | 29:30 | Septa spacing 3 | 5:7 |
| Septa length 4 | 7:8 | Septa width 4 | 31:32 | Septa spacing 4 | 7:9 |
| Septa length 5 | 9:10 | Septa width 5 | 33:34 | Septa spacing 5 | 9:11 |
| Septa length 6 | 11:12 | Septa width 6 | 35:36 | Septa spacing 6 | 11:13 |
| Septa length 7 | 13:14 | Septa width 7 | 37:38 | Septa spacing 7 | 13:15 |
| Septa length 8 | 15:16 | Septa width 8 | 39:40 | Septa spacing 8 | 15:17 |
| Septa length 9 | 17:18 | Septa width 9 | 41:42 | Septa spacing 9 | 17:19 |
| Septa length 10 | 19:20 | Septa width 10 | 43:44 | Septa spacing 10 | 19:21 |
| Septa length 11 | 21:22 | Septa width 11 | 45:46 | Septa spacing 11 | 21:23 |
| Septa length 12 | 23:24 | Septa width 12 | 47:48 | Septa spacing 12 | 23:1 |
| Theca thickness 1 | 1:49 | Corallite width 1 | 1:13 | Corallite spacing 1 | 61:62 |
| Theca thickness 2 | 3:50 | Corallite width 2 | 3:15 | Corallite spacing 2 | 61:63 |
| Theca thickness 3 | 5:51 | Corallite width 3 | 5:17 | Corallite spacing 3 | 61:64 |
| Theca thickness 4 | 7:52 | Corallite width 4 | 7:19 | Corallite spacing 4 | 61:65 |
| Theca thickness 5 | 9:53 | Corallite width 5 | 9:21 | Corallite spacing 5 | 61:66 |
| Theca thickness 6 | 11:54 | Corallite width 6 | 11:23 | Corallite spacing 6 | 61:67 |
| Theca thickness 7 | 13:55 | Fossa width 1 | 2:14 | Corallite spacing 7 | 61:68 |
| Theca thickness 8 | 15:56 | Fossa width 2 | 4:16 | Corallite spacing 8 | 61:69 |
| Theca thickness 9 | 17:57 | Fossa width 3 | 6:18 | ||
| Theca thickness 10 | 19:58 | Fossa width 4 | 8:20 | Corallite Area | 1:3:5:7:9:11:13:15:17:19:21:23 |
| Theca thickness 11 | 21:59 | Fossa width 5 | 10:22 | Fossa Area | 2:4:6:8:10:12:14:16:18:20:22:24 |
| Theca thickness 12 | 23:60 | Fossa width 6 | 12:24 |
Table 1. Summary of which points are used for the calculation of various corallite microstructures.
EXAMPLE: To calculate the length of the dorsal septa (aka "Septa Length 1") we will use the X and Y coordinates of Point #1 and Point #2 (recall Table 1) which were measured and are shown in Figure 14.
- Point 1 (X = 2.869, Y = 2.914)
- Point 2 (X = 3.248, Y = 2.991)
Next, using the equation shown in Step 21:
- Septa Lenth 1 = √ [ (3.248 – 2.869)2 + (2.991 – 2.914)2]
Therefore, Septa Length 1 = 0.387 cm
In addition to distances between points, the area of polygons constructed from multiple points can also be calculated. For instance, Corallite Area or Fossa Area (cm^2) can be calculated using the following equation: