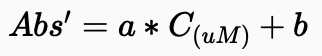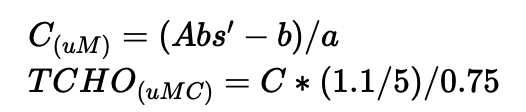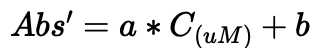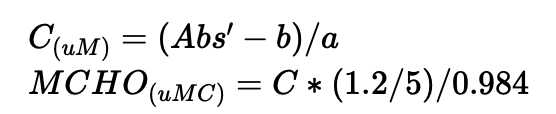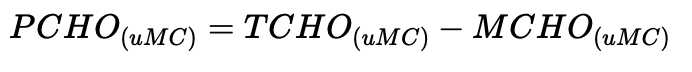Measurement of dissolved carbohydrate
Ying-Yu Hu, Zoe V. Finkel
Dissolved total carbohydrate
Dissolved polysaccharide
Dissolved monosaccharide
TPTZ method
Ferricyanide
hydrolysis
Abstract
Here we describe a protocol to measure the dissolved carbohydrate, including total dissolved monosaccharides and total dissolved polysaccharides.
For total dissolved carbohydrate measurement, freeze-dried dissolved carbohydrate samples are initially vortexed in 9 M H2SO4 for 15 s. The solution is diluted for a final H2SO4 molarity of 1.6 M and hydrolyzed for 3 hours at 90 °C. The hydrolysate is alkalinized by adding 12 M NaOH to the hydrolysate, the ratio of [H+] from hydrolysate to [OH-] from NaOH is 0.82. The alkalinized hydrolysate is oxidized by ferricyanide solution. The absorbance of TPTZ-Fe2+ complex is measured in microtiter plate at 595 nm.
For total dissolved monosaccharide measurement, freeze-dried dissolved carbohydrate samples are alkalinized by 12 M NaOH and then oxidized by ferricyanide solution. The absorbance of TPTZ-Fe2+ complex is measured in microtiter plate at 595 nm.
Our method has shown high reproducibility in aldohexoses, ketohexoses, deoxysugars, aldopentoses, uronic acid and amino sugars. The low limit of detection is 0.024 µg C/mL.
Steps
Sample collection
GFF filter is combusted for 4h 0m 0s at 450°C
Glass filter holder is combusted for 2h 0m 0s at 500°C
Glass filter funnel, flask and 10 mL centrifuge tubes are combusted for 6h 0m 0s at 500°C
Equipment
| Value | Label |
|---|---|
| Disposable Glass Screw-Cap Centrifuge Tubes | NAME |
| 10 mL | TYPE |
| Corning® | BRAND |
| 99502-10 | SKU |
Tube caps are acid-washed.
Equipment
| Value | Label |
|---|---|
| Polypropylene Screw Caps | NAME |
| Linerless, 15-415 | TYPE |
| Kimble Chase | BRAND |
| 73805-15415 | SKU |
Filter microalgae sample and collect the filtrate, using gentle vacuum pressure (130 mm Hg).
Transfer 5 mL filtrate into centrifuge tube and flash freeze.
Freeze dry samples before measurement.
Glucose standards
Primary standard solution
In a 2 mL microtube, weigh 1 ~ 2 mg D-glucose
Add Milli-Q for a final concentration of 1 mg/mL (>600 µL).
Secondary standard for total dissolved carbohydrate
Add 45µL primary solution into a 2 mL microtube
Add 955µL Milli-Q and then vortex for a good mix
In 10 mL centrifuge tubes, prepare the following standard solutions:
| A | B | C |
|---|---|---|
| TCHO-SD1 | 0 | 100 |
| TCHO-SD2 | 20 | 80 |
| TCHO-SD3 | 40 | 60 |
| TCHO-SD4 | 60 | 40 |
| TCHO-SD5 | 80 | 20 |
| TCHO-SD6 | 100 | 0 |
Secondary standard for total dissolved monosaccharide
Add 10µL primary solution into a 2 mL microtube
Add 990µL Milli-Q and then vortex for a good mix
In 12 mL amber vials, prepare the following standard solutions:
| A | B | C | D |
|---|---|---|---|
| MCHO-SD1 | 0 | 984 | 16 |
| MCHO-SD2 | 10 | 974 | 16 |
| MCHO-SD3 | 20 | 964 | 16 |
| MCHO-SD4 | 50 | 934 | 16 |
| MCHO-SD5 | 100 | 884 | 16 |
| MCHO-SD6 | 150 | 834 | 16 |
Equipment
| Value | Label |
|---|---|
| Storage Vials and Closures | NAME |
| 12 mL amber | TYPE |
| Thermo Scientific | BRAND |
| B7800-12A | SKU |
| VWR 66030-686 | SPECIFICATIONS |
Hydrolysis of total dissolved carbohydrate
Prepare water bath 95°C
Add 100µL Milli-Q to each tube with freeze-dried sample.
Use reverse pipetting technique, add 100µL 18 M H2SO4 to standard solution/sample, immediately vortex for 0h 0m 15s (monitored by timer or stopwatch)
Add 900µL Milli-Q, tightly cap the centrifuge tube, and vortex for 0h 0m 5s .
Place tube into water bath, log the time.
After all samples are in the water bath, reduce temperature to 90°C .
Label amber vials for TPTZ measurement with white oil based sharpie.
# of vials = # of samples + # of blanks + # of standards
As soon as hydrolysis duration reaches 3 hours, remove the tube from water bath, let it sit in the tap water bath with ice to quickly stop hydrolysis.
Keep all hydrolysate in a dark cabinet at Room temperature .
Prepare TPTZ reagents
12 M NaOH
Add 15 mL Milli-Q water into a 50 mL Falcon tube.
Add 12g NaOH pellet into the water, swirl and have the pellets completely dissolved, let it cool down to Room temperature .
Transfer the solution into a 25 mL PP volumetric flask, rinse the tube three times by small amount of Milli-Q and combine the rinsed water into flask, top with Milli-Q water to 25 mL.
Alkaline solution for potassium ferricyanide
Dissolve 400mg NaOH and 20g Na2CO3in volumetric flask and top to 1 L by Milli-Q. Store at room temperature.
Sodium acetate solution
Dissolve 164g sodium acetate, 42g citric acid and 300g acetic acid in a 1 L volumetric flask and top to1 L with Mill-Q water.
Store at room temperature.
Dispense solution by serological pipet to avoid having salt precipitated around sealing surface of the bottle.
3 M acetic acid
Weigh 180g acetic acid in fumehood, transfer the acid into volumetric flask, top to 1 L with Milli-Q water. Store at room temperature.
TPTZ method
Prepare boiling bath
TPTZ reagents
Potassium ferricyanide (Reagent A)
Weigh 23mg potassium ferricyanide and transfer into a 100 mL amber reagent bottle. Add 100mL alkaline solution, vortex until powder is completely dissolved. It is stable for two weeks at room temperature.
Equipment
| Value | Label |
|---|---|
| Reagent bottle | NAME |
| 100 mL, amber | TYPE |
| VWR | BRAND |
| 14216-240 | SKU |
Ferric chloride (Reagent B)
Ferric chloride hexahydrate is in spherical shape. It is hard to weigh exact 54 mg for a 100 mL solution. Pick a very small ferric chloride ball and log the weight. Transfer the ball into a 100 mL amber reagent bottle. Calculate the acetate solution required. Add acetate solution into the amber bottle, vortex until the ball is completely dissolved.V_acetate = 100 X W_actual/54
TPTZ (Reagent C)
Estimate the total volume required for the assay: 2 mL X (standard # + blank # + sample #)
For each 100 mL TPTZ reagent, weigh and transfer 78 mg TPTZ into an amber reagent bottle, add 100 mL acetic acid solution, vortex until the powder is completely dissolved.
Total dissolved carbohydrate samples
Use reverse pipetting technique, transfer 750µL hydrolysate of standard/sample to amber vial.
Add 250µL 12 M NaOH and vortex.
Total dissolved monosaccharide samples
Add 1200µL Milli-Q into the tube with freeze-dried sample
Use reverse pipetting technique, transfer 984µL solution to amber vial.
Add 16µL 12 M NaOH and vortex.
In a room with dim light, add 1mL Reagent A into each amber vial.
Tightly cap the vial and vortex.
Keep in a boiling water bath for 0h 10m 0s
Remove boiling bath from the heat, keep all vials in the hot water and move them into the room with dim light.
Add 1mL Reagent B and 2mL Reagent C into the vial and vortex.
Shake at Room temperature for 0h 30m 0s .
Under dim light, using reverse pipetting, load 250 uL of blanks, standards, and samples into the microplate (duplicate).
Load column by column. After one column has been loaded, immediately cover the column with a lid, which has a black membrane on the top to protect sample from light.
| A | B | C | D | E | F | G | H | I | J | K | L | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | MCHO-SD1 | MCHO-SD1 | ||||||||||
| B | MCHO-SD2 | MCHO-SD2 | ||||||||||
| C | MCHO-SD3 | MCHO-SD3 | ||||||||||
| D | MCHO-SD4 | MCHO-SD4 | ||||||||||
| E | MCHO-SD5 | MCHO-SD5 | ||||||||||
| F | MCHO-SD6 | MCHO-SD6 | ||||||||||
| G | ||||||||||||
| H |
Microplate layout for dissolved monosaccharide samples
| A | B | C | D | E | F | G | H | I | J | K | L | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | TCHO-SD1 | TCHO-SD1 | ||||||||||
| B | TCHO-SD2 | TCHO-SD2 | ||||||||||
| C | TCHO-SD3 | TCHO-SD3 | ||||||||||
| D | TCHO-SD4 | TCHO-SD4 | ||||||||||
| E | TCHO-SD5 | TCHO-SD5 | ||||||||||
| F | TCHO-SD6 | TCHO-SD6 | ||||||||||
| G | ||||||||||||
| H |
Microplate layout for total dissolved carbohydrate samples
Read in microplate reader:Shake for 5 s at 600 rpm in a continuous and high force modeRead endpoint 595 nm with a measurement time 100 ms
UV/VIS spectra (optional)
Hydrolysate
Load 200µL hydrolysate into microplate.
Blank:
Milli-Q : H2SO4= 10:1
Monosaccharide solutions
Load 200µL solution into microplate.
Blank: Milli-Q
Scan UV/VIS spectra from 200 to 400 nm at a step of 1 nm.
Calculation
Total dissolved carbohydrate
Subtract the average absorbance of blank (0 ug glucose) from the absorbance of each standard for total dissolved carbohydrate.
Subtract the average absorbance of blank (0 ug glucose) from the absorbance of each sample
Total dissolved monosaccharide
Subtract the average absorbance of blank (0 ug glucose) from the absorbance of each standard for total dissolved monosaccharide.
Subtract the average absorbance of blank (0 ug glucose) from the absorbance of each sample
Waste disposal
All hydrolysate and TPTZ reagent C need to be neutralized by soda before disposed into the sink.
TPTZ reagent B is collected in trace metal waste container.