Isotopically labelled inorganic carbon delivered to algal cultures via bubbler bottle
Sunnyjoy Dupuis, Usha F Lingappa, Sabeeha S. Merchant, Xavier Mayali
stable isotope probing
algae
bicarbonate
carbonate chemistry
bubbling
chlamydomonas reinhardtii
photosynthesis
Abstract
This protocol describes a method for delivering labelled inorganic carbon as 13CO2 to algal cultures by bubbling air through a solution of H13CO3-, and then into the culture. We developed this method to deliver label to cultures grown under continuous bubbling with air, without the use of 13CO2 labelled gas and the necessary equipment to mix labelled gas with air at near-atmospheric levels. Bubbling precludes the more common approach of adding H13CO3- label directly to the media, because dissolved HCO3- is in equilibrium with atmospheric CO2. Thus, excess HCO3- added to a solution will leave the solution as CO2 gas as it equilibrates. Bubbling rapidly accelerates this equilibration which is typically diffusion limited. The method described here takes advantage of this aspect of carbonate chemistry, and uses a solution of H13CO3-—which is less expensive and more convenient than 13CO2 gas—to generate a flux of 13CO2 that can be bubbled into a culture.
Steps
The purpose and problem of bubbling cultures
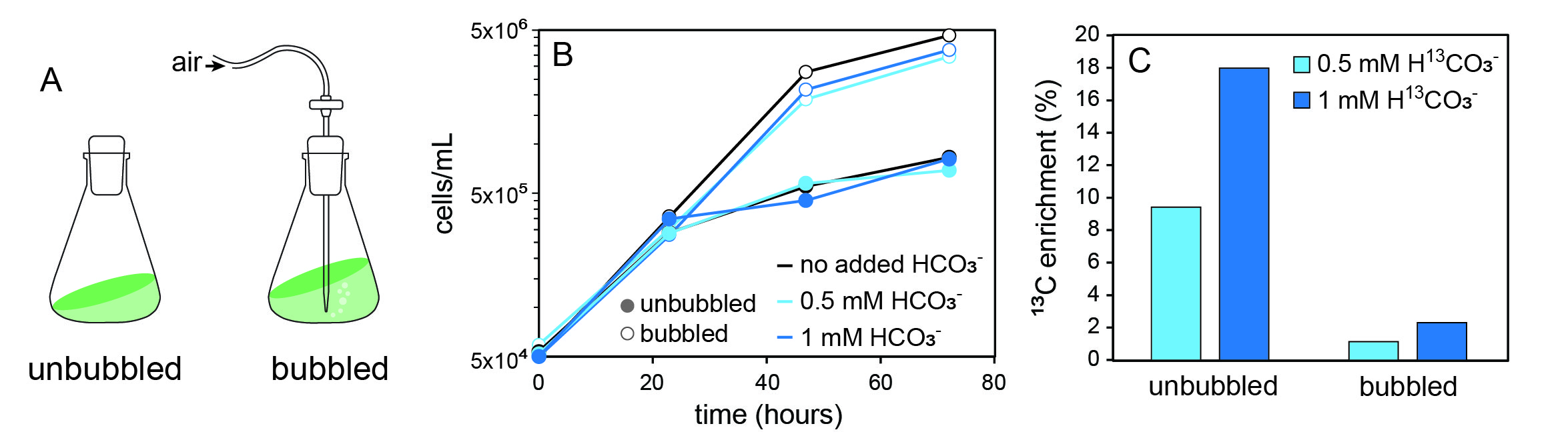
Bubbling air into algal cultures stimulates photosynthetic growth by ameliorating diffusion limitation for CO2 (fig. 1A-B). Stable isotope probing (SIP) experiments examining carbon fixation often involve 13C label introduced directly into the culture medium as a H13CO3- salt. Unfortunately, because dissolved inorganic carbon (DIC) is in equilibrium with atmospheric CO2 (eq. 1), this labelling method does not work for cultures that are bubbled in an open system. Bubbling accelerates equilibration, causing excess HCO3- to rapidly exit the solution so that the label is lost before it can be fixed into biomass (fig. 1C). This presents some inconvenience, as H13CO3- salts are cheaper and easier to work with than is 13CO2 gas.
Equation 1. CO2(g) + H2O ⇋ H2CO3 ⇋ HCO3- + H+ ⇋ CO32- + 2H+ 2(g) + H2O ⇋ H2CO3 ⇋ HCO3- + H+ ⇋ CO32- + 2H+
Label delivery via bubbler bottle
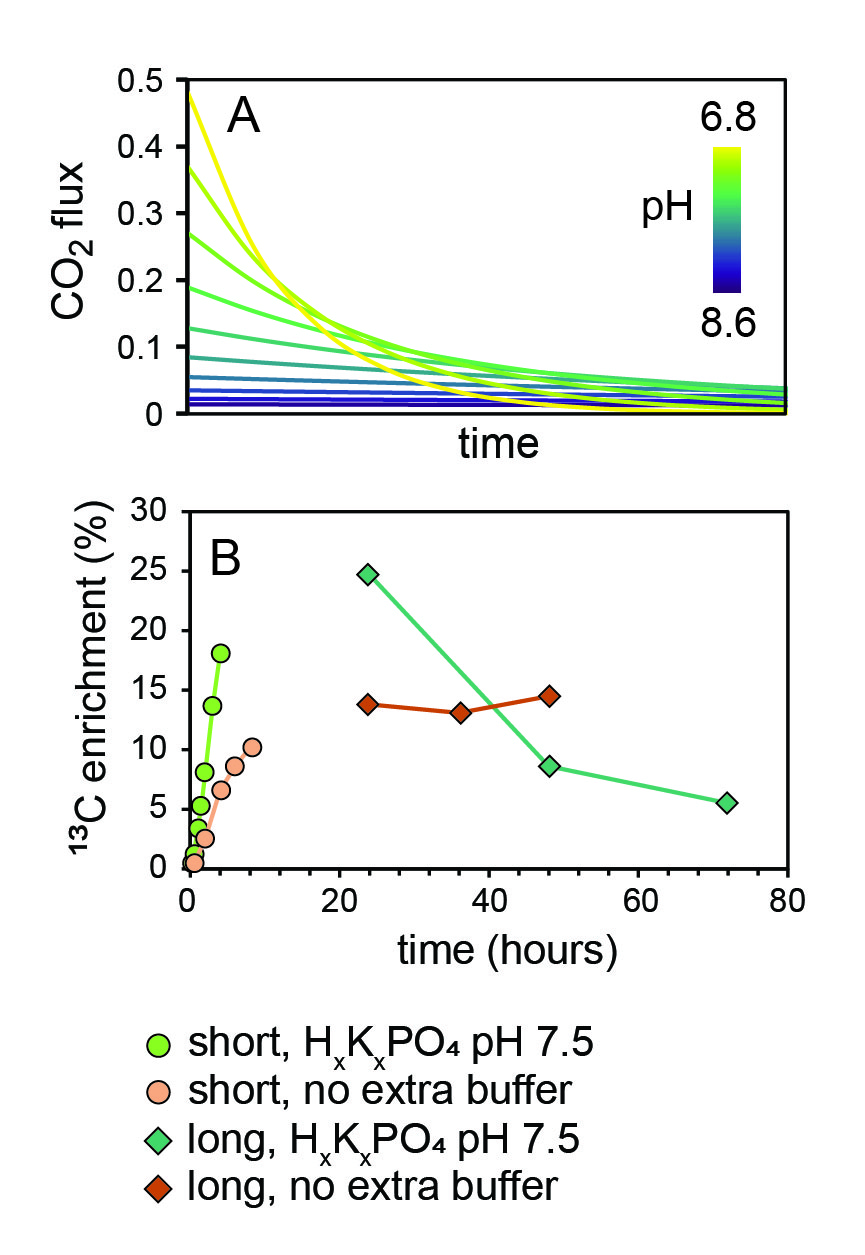
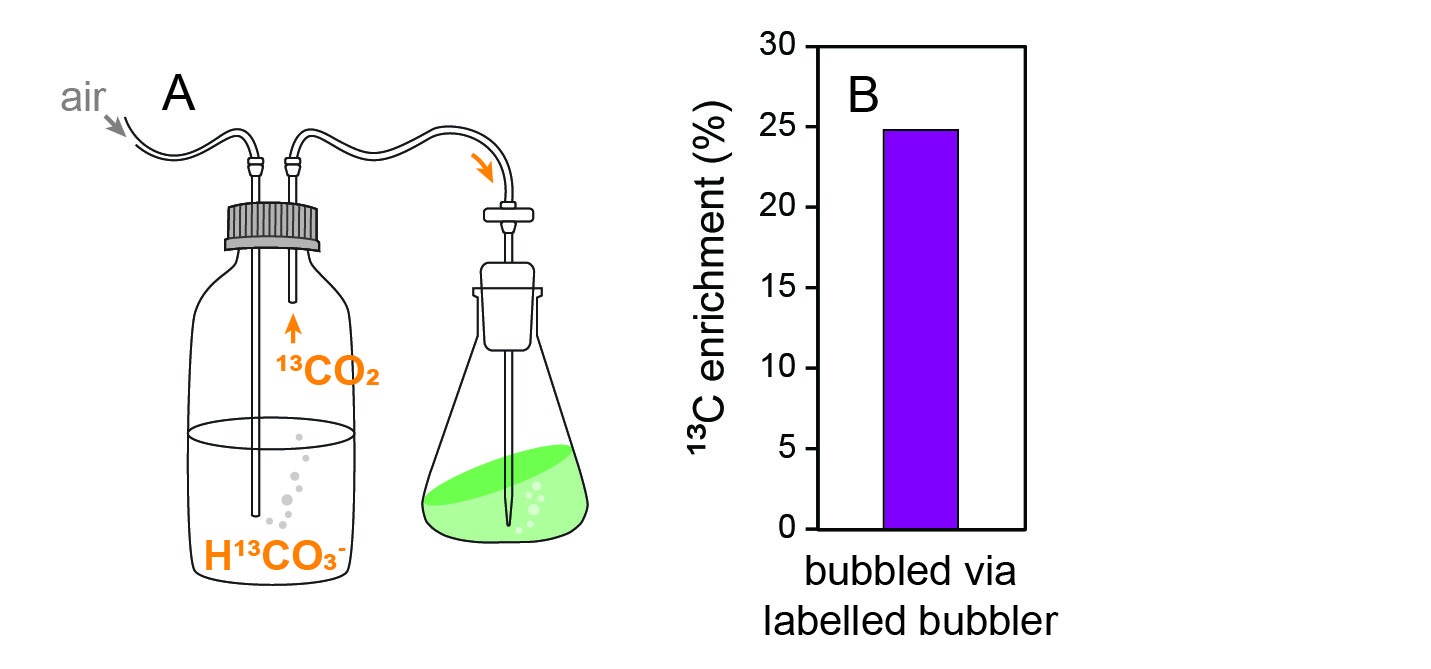
We developed a SIP method that takes advantage of this liability of bubbling and carbonate chemistry (figure 2). Using an aquarium pump, we bubble air into a solution of H13CO3-. The DIC in the solution exchanges with the air, releasing label in the form of 13CO2 gas. That air is then bubbled into algal cultures. Using this method, we achieved substantial biomass 13C enrichment in bubbling C. reinhardtii cultures (fig. 2B).
The rate of label release in this method can be tuned by buffering the bubbler solution at different pH values (fig. 4A). In lower pH solutions, carbonate equilibria (eq. 1) shift towards a higher fraction of the DIC pool speciated as dissolved CO2, which increases the rate at which that DIC exchanges into the bubbled air. We identified solution conditions suitable for experiments requiring rapid incorporation of 13C into algal biomass over short timescales (minutes) and for steady-state release over longer timescales (days). With a bubbler solution buffered with phosphate at pH 7.5, we obtained 13C enrichment detectable in algal biomass by IRMS in <15 minutes (fig. 4B, green). With a bubbler solution devoid of any additional buffer beyond the HCO3- itself, we obtained slower but longer-lived steady-state incorporation of the label (fig. 4B, orange).