Intrahepatic implantation of tumor cells
donna.peehl, Renuka Sriram, Shubhangi Agarwal, Robert Bok
Abstract
This protocol describes the steps required for successful implantation of small cell neuroendocrine prostate cancer patient-derived xenograft (PDX) cells in the liver. Liver is one of the most common sites for development of metastatic prostate cancer and its study is important for evaluating the tumor characteristics and response to therapy.
This protocol can be used for implantation of any tumor cell line in the liver.
Steps
Preparation before surgery
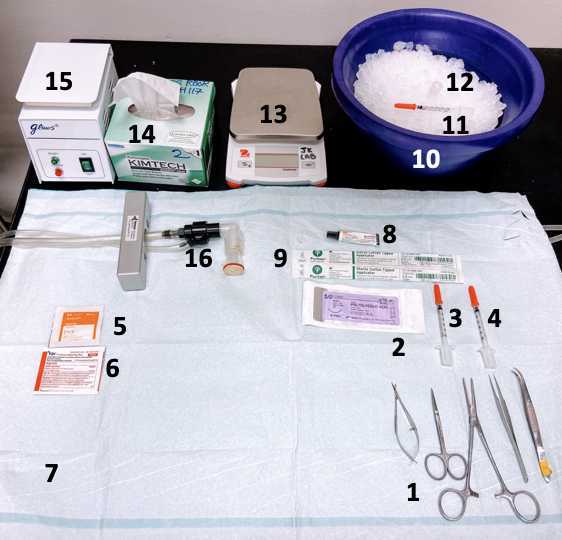
Preparation of surgical instruments and supplies: All of the instruments and supplies should be sterilized.
Surgery record sheets
| A | B | C |
|---|---|---|
| PI | ||
| Personnel | ||
| Date | ||
| Procedure name | Hepatic Tumor Implantation | |
| Protocol # | ||
| Type | Survival Surgery | |
| Species | Mouse | |
| Experimental agents administered | Tumor cells | |
| Anesthetics | Isoflurane | Dosage: (1-5 % or mg/kg; mL) |
| Analgesics | (1) Lidocaine | Dosage |
| (2)Buprenorphine | Dosage | |
| A | B | C |
|---|---|---|
| Mouse ID | ||
| Mouse weight | ||
| Anesthesia start time | ||
| Analgesics | (1) Lidocaine | Time administered: |
| (2) Buprenorphine | Time administered: | |
| Tumor cells administration time | ||
| Anesthesia end time |
Preparation of the PDX cells for implantation
Preparation of cells for implantation from frozen biobank
Prepare DMEM medium: Prepare fresh DMEM media supplemented with
10% volume FBS and
100ug/ml Gentamicin.
Retrieve cryovials containing cells from liquid nitrogen storage. Thaw the cell-containing cryovial by placing it in 37 degrees water bath. Move the vial into a BSL2 hood and transfer the contents of the vial in a 15 ml conical tube containing fresh DMEM media (9mL DMEM per 1mL of the cell mixture) and mix gently.
Perform live/dead assay using trypan blue and note the live and total cell count.
Centrifuge the 15 ml tube containing cells at 300x g,0h 0m 0s and aspirate the supernatant. Resuspend the cells in fresh DMEM to a final concentration of 1M live cells/10-20 ul and transfer this mixture into an eppendorf tube.
Transfer the cell suspension into a syringe right before beginning the surgery.
Preparation of surgical space
Place a disposable sterile pad on a heating pad.
Aseptically sterilize the surgical area by spraying with 70% ethanol.
Station 2: Surgery
Place a disposable sterile pad on a heating pad.
Aseptically sterilize the surgical area by spraying with 70% ethanol.
Place all the autoclaved surgical instruments within the sterilized surgical area.
Anesthetization and fur removal of mouse
Place the animal in a knock-down box circulating with a gas mixture of Isoflurane @ 1.4-2.0% and O2 @ 1-1.2 lt/min inhalant, maintained via a bain-closed system.
Note the start time for anesthesia.
Move the animal to the station 1 nose cone and apply ophthalmic ointment on the animal’s eyes to prevent them from drying out during the procedure.
Determine the anesthetic depth by pinching the animal’s foot for a reflex response.
Disinfect the surgical area by rubbing the area in a circular motion with a povidone-Iodine prep pad followed by a 70% alcohol pad. Repeat this process 2 more times.
The animal is now ready to be moved to station 2 for surgery.
Surgical procedure (Station 2)
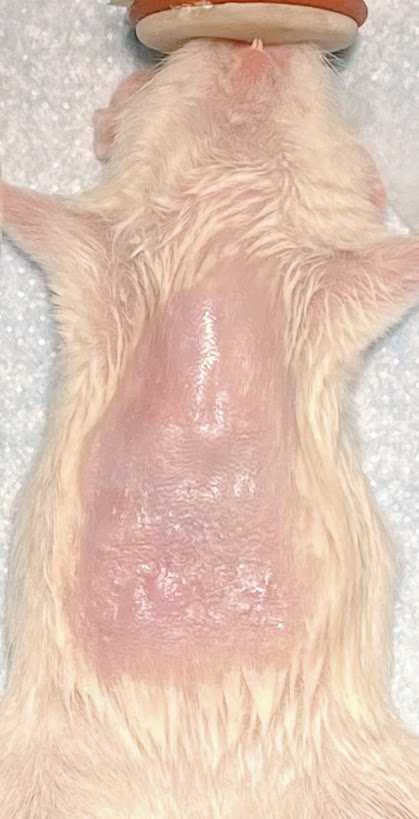
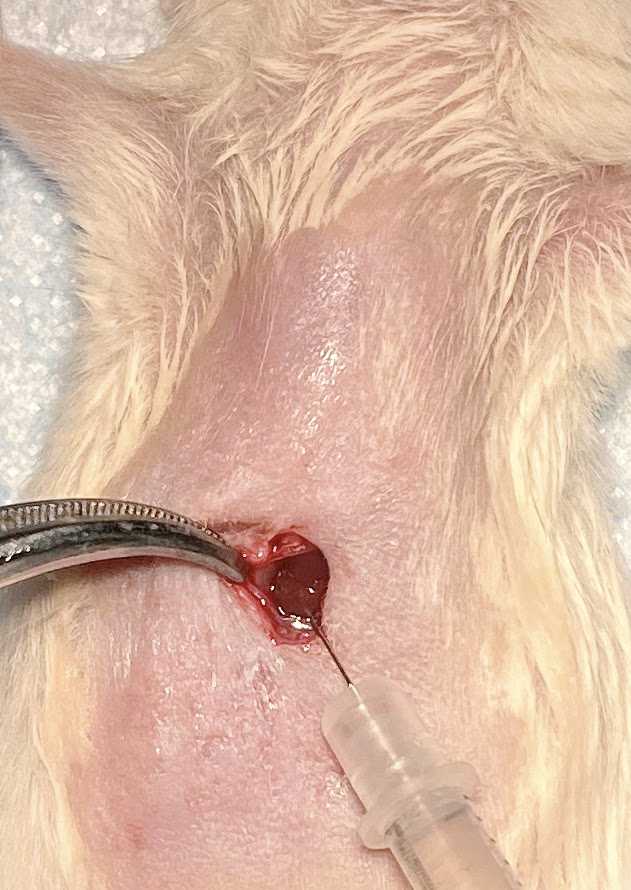
Gently grab the peritoneum muscle layer and make an incision to expose the liver.
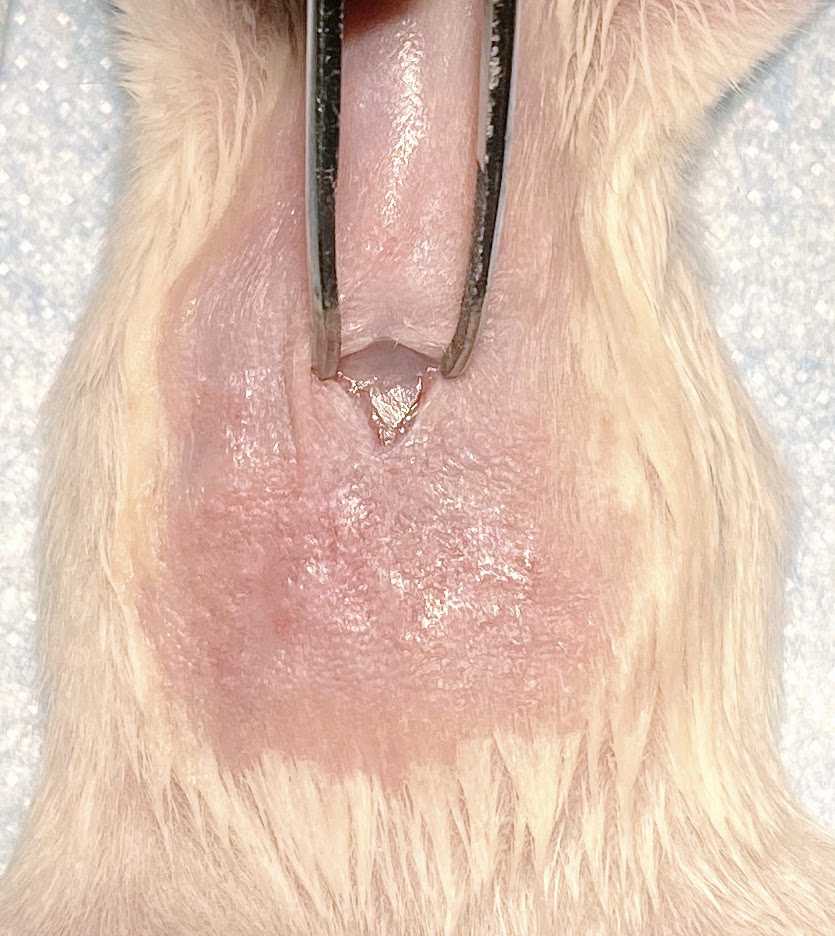
Transfer the cell suspension into a 1 cc syringe with a 27 G needle.
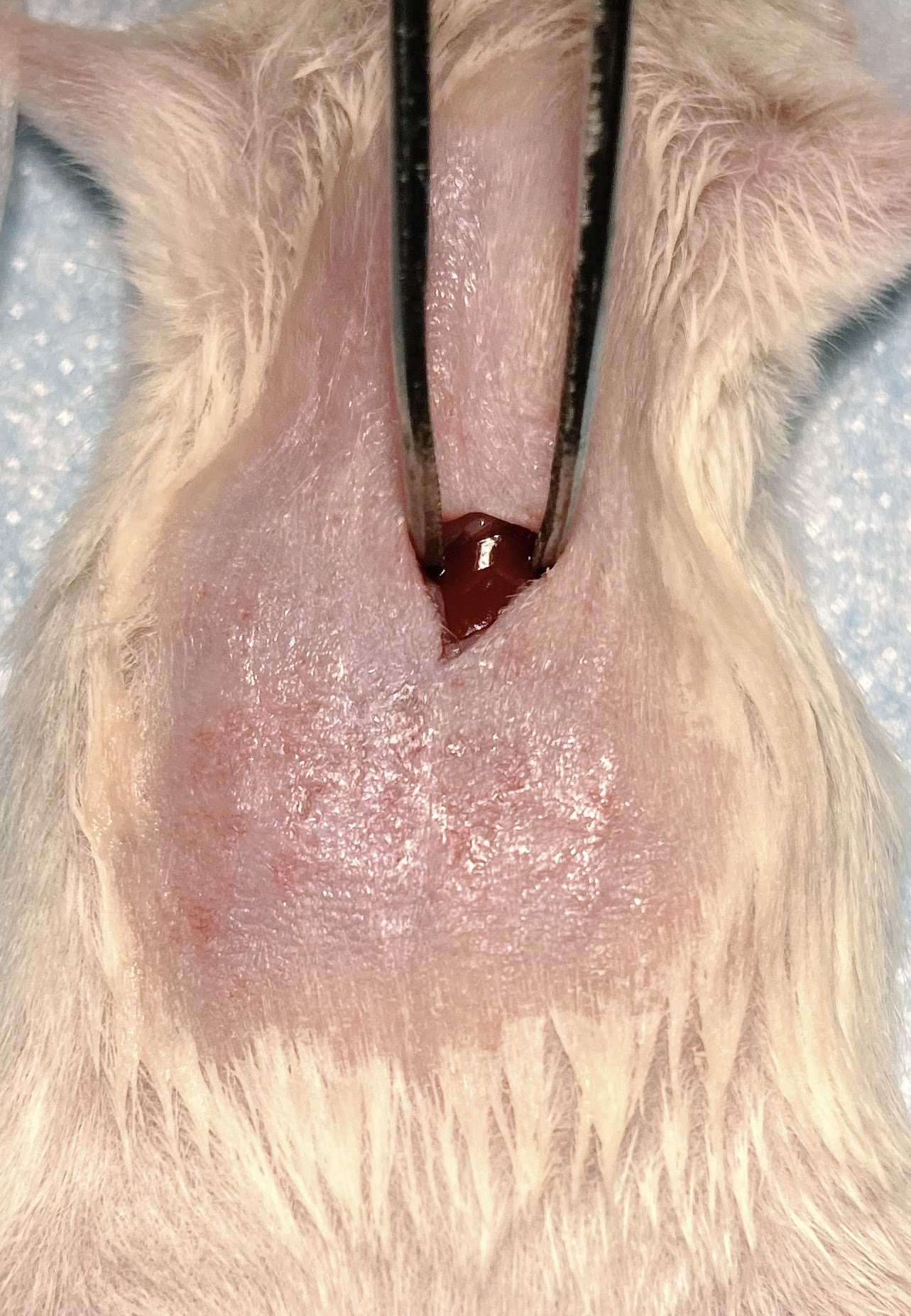
Inject 10-20 ul of cells suspension from the syringe into the liver. Hold the syringe in place for 20-30 secs to minimize any leakage of cells from the liver.
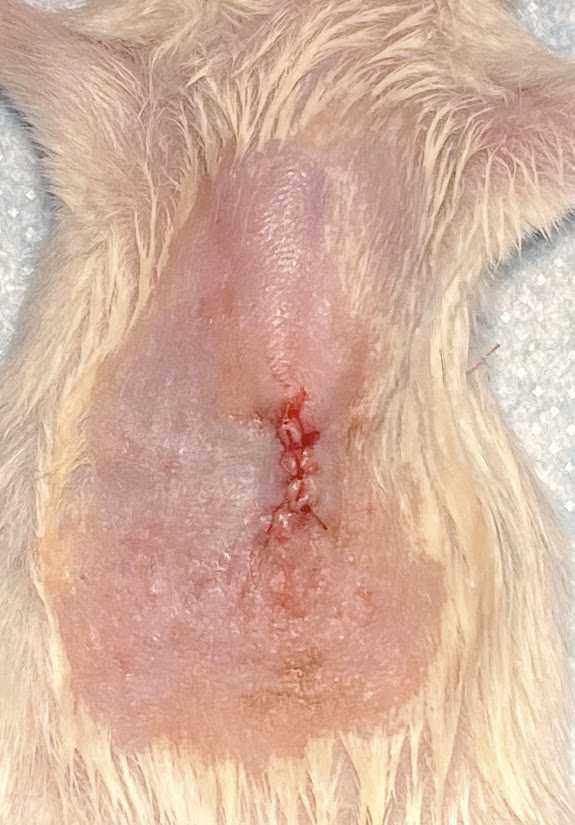
Pull the needle slowly while applying pressure with sterile cotton-tipped applicators in order to minimize leakage of the cells. The injection site should turn white upon successful injection.
Upon completion of the sutures administer buprenorphine and lidocaine subcutaneously. Note the time of administration.
Sterilize the surgical instruments using the bead sterilizer by placing them inside for ~ 10 secs.
Start prepping the next mouse for surgery.
Post-Op care and monitoring
Place the animal in a clean cage on a warm heating pad to aid in regaining its body temperature.
Observe the animal until it has regained full consciousness and is walking around in the cage.
Observe the animal on a daily basis until sacrificed as per your IACUC guidelines.