Intensive education intervention using network of involved diabetes patients to improve glycaemic control of type 2 diabetes patients in Delta State Nigeria: study protocol for a randomised controlled trial
Victor M Oguoma, Ezekiel U Nwose, Eunice O Igumbor, Charles C Ofili, Phillip T Bwititi, Adeseye A Akintunde, Ifeoma I Ulasi, Echinei J Oshionwu, John Okuzor, Alex Odufu, Kennedy Aninze, Emmanuel Olu-Ero, Timothy C Skinner
Abstract
Background: There is lack of structured self-management of type 2 diabetes (T2D) available in Nigeria. Therefore, we sought to determine if T2D patients receiving additional intensive lifestyle education intervention within a network of T2D patients compared to those receiving the usual secondary healthcare education at 12 months will have better glycaemic control.
Methods: This open-label, parallel, two-arm randomised control trial will enrol 180 individuals (90 per group) to either usual healthcare education or complementary intensive peer education using network of T2D patients at baseline and followed to 12 months. Individuals between 18-60 years of age with T2D and are willing to undergo the process of peer education are eligible. Our primary outcome is the absolute change in mean HbA1c from baseline to 12 months. Secondary outcomes will include change in weight, CVD risk profile, social support, stress level, and quality of life.
Discussion: There is limited evidence on the effectiveness of peer education in the management of T2D within the low-income setting such as Nigeria. Therefore, this trial will contribute to the evidence on effectiveness of patient-led intensive peer education in diabetes self-management for T2D patients.
Trial registration : PACTR201811637528789 (7/11/2018)
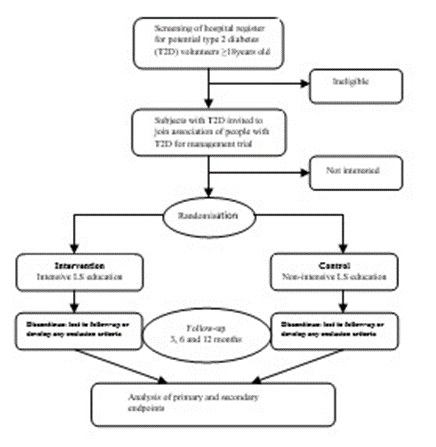
Steps
BACKGROUND
Sub-Sahara Africa (SSA) is the region in the world with the highest number of individuals with undiagnosed type 2 diabetes (T2D), who are thus at very high risk of developing multiple chronic complications due to low awareness and lack of management of the condition [1, 2]. Nigeria, the most populous of African countries is the third largest country in SSA with people suffering from T2D [3]. Incidence and prevalence of CVD is still unclear in Nigeria. Reports from hospitalised patients and archived death records show a younger age of onset [4, 5], implicating huge co-morbidity of hyperglycaemia, with other CVD risk factors as contributors to the epidemiological transition.
In 2015, there were more than 1.56 million cases of diabetes in Nigeria [3]. The only national survey over two decades ago reported 2.8% (95% CI 2.6 – 3.1%) prevalence of diabetes [6]. However, recent population-based cross-sectional observational studies report between 3.3 – 8.4% prevalence of T2D [7-9]. Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) exist in greater proportions in concurrence with other CVD risk factors more than that identified in overt T2D populations [10, 11].
Lifestyle modification intervention studies or programs in the management of T2D and CVD in the dynamic Nigerian population is limited. Health services for prevention have been mainly provided by medical screening outreaches organized by researchers and non-governmental organizations (NGOs), while treatment is largely inadequate due to expensive healthcare services and focus on management of overt conditions. This stretches the fragile health systems which has been overrun by high prevalent morbidity and mortality from infectious diseases. The benefits of lifestyle modification programs have been established in studies elsewhere [12, 13], and its level of efficacy in preventing T2D and CVD can last up to 14 years after termination of lifestyle modification [14, 15].
In our ongoing research, we have evaluated how age, prevalence of diabetes-related chronic diseases and intercurrent illnesses may be influencing lifestyle. Part of our findings was that the effect of gender and age on total physical activity level is quite significant, but not in the five different domains of physical activity and sedentary time [16]. That is, considering daily activities as a form of exercise that improve health, the interference of ill-health on going to work, social activities, recreation, household and outside chores increases with age, particularly in women.
This can be translated to emphasize a potential significance of sedentary lifestyle change to investigate, but in the context of age and intercurrent illness intervening daily activities. We had reported that while concerted efforts to improve physical activity are required, metabolic syndrome may not be improved by being physically active alone [17]. Hence, we emphasize on how daily activities significantly decreased with increasing age-groups. That is, aging, as a physiological process, intervenes on ability for routine physical activities enroute interference with lifestyle and associated morbidities; while intercurrent illness is equally prevalent across all age-groups and may only constitute confounding interference on lifestyle in older adults. What has yet to be evaluated in our research program is the effectiveness of intensive ‘lifestyle’ education intervention using peer educators. Thus, there is still the community assessed need of how best to employ education and lifestyle changes in management of diabetes in the target population.
STUDY OBJECTIVES
- The primary objective of the trial is to evaluate whether T2D patients that undergo intensive lifestyle education within a network of T2D patients as peer-educators compared to usual lifestyle education will have better blood glycated haemoglobin (HbA1c) at 12 months of intervention.
- The four secondary objectives include:
- To assess whether T2D patients with access to intensive lifestyle education within a network of T2D patients as peer-educators will have reduced weight compared to those attending usual lifestyle education at 12 months.
- To assess whether the cardiovascular diseases risk profile of T2D patients with access to intensive lifestyle education within a network of T2D patients as peer-educators will improve compared to those attending usual lifestyle education at 12 months.
- To determine if there will be improved social support and reduced diabetes stress amongst participants attending intensive peer-supported lifestyle education as compared to those in usual lifestyle education at 12 months.
- To determine the quality of life of T2D patients under peer-supported intensive lifestyle education in comparison to those under usual care at 12 months.
METHODS
Trial design, ethics and setting
Trial design
The trial is an open-label, parallel, two-arm randomised control trial with two groups (1:1), to compare the effectiveness of an intensive lifestyle education intervention within a network of T2D patients as peer-educators, compared to those receiving the usual care at 12 months of follow-up. The project was launched with a kick-off information workshop in the week of June 2018, while the trial commenced in November 2018.
Study setting
The trial was conducted at Novena University as the administrative site, and five health facilities as performance sites with the Catholic Hospital Abbi (CHA) as the primary healthcare site. Abbi is one of the major rural towns of Ndokwa-West local government area in Delta state of Nigeria. The town is about 100 km from the state capital; and approximately 30 km from the nearest General Hospital, which is located at the local government headquarters in Kwale. The selection of CHA as primary performance site was based on the ongoing international diabetes research program of Global Medical and Research Development Organization with multidisciplinary team with high level skills in peer education projects.
Ethics, consent, and dissemination
The study has been approved by the Research Ethics Committee of Novena University and the Delta State Ministry of Health (HM/596/T/197). Trained research staff will undertake informed assent and consent. Information was provided to study participants in writing. Signed consent was required at the time of randomisation when eligibility criteria were confirmed. The consent process will include explanations of all elements of consent, according to the Declaration of Helsinki on ethical principles for medical research involving human subject.
Recruitment
| A | B | C | D | E |
|---|---|---|---|---|
| Visit No. | 0 | 1 | 2 | 3 |
| Event | Screening/baseline | 3 months | 6 months | 12 months |
| Informed consent | x | |||
| Randomisation | x | |||
| Anthropometric measurements | x | x | x | x |
| Blood assessment (HbA1C, FBG, and Lipid profile) | x | x | x | x |
| Blood pressure assessment | x | x | x | x |
| General health | x | x | x | x |
| Daily activity | x | x | x | x |
| Physical activity and diet | x | x | x | x |
| Perceived susceptibility to diabetes complications | x | x | x | x |
| Perceived severity of diabetes mellitus | x | x | x | x |
| Perceived benefits of adherence to diabetes treatment guidelines | x | x | x | x |
| Perceived barriers to prescribed diabetes treatment | x | x | x | x |
| Nutrition and exercise self-efficacy | x | x | x | x |
| Patient health | x | x | x | x |
| Multidimensional scale of perceived social support | x | x | x | x |
| Quality of life | x | x | x | x |
Table 1. Schedule of assessments
Details of data collection at each of the scheduled assessment times are summarised in Table 1. Persons targeted by the project was recruited through the hospitals that have been supporting the international research collaboration. Based on the diabetes register created at various health facilities, volunteers among the known diabetes patients were invited through their health facility contacts to attend a diabetes outreach. At the different geographical locations (i.e. based at health facilities), networks association was formed among them in order to foster interaction as per agenda for peer-education and support, which was the first step in developing association/network of diabetic patients for peer-education. This will represent an important link between patients, educators, and general medical services.
Healthcare workers were trained on the theoretical frameworks of diabetes self-management (DSM) [18, 19]; and peer-education [20, 21]. The training was conducted using collaborative and reflective learning pedagogies in order that they can train the T2D patients, who will form networks of T2D peer-educators. Training handbook [22], as well as educational posters and soft-bound booklets were produced to facilitate the training. Secondly, peer-educators were recruited from the volunteer T2D patients and trained using collaborative and reflective learning. Educational booklets, leaflets and posters were provided. Thirdly, the intensive peer-education intervention involved learning nests format of training and practical demonstrations. Where applicable, such as during ‘nutrition and cooking’ and physical activity sessions, in real-life scenarios were adopted. The training peer-educators, and subsequently participants in intervention group, will include four (4) educational sessions: control of cardiovascular risk factors, dietary control, physical activity and medications. The last session on medications included issues of insulin as well as diabetes self-management plans. Patients were led to develop self-management programs through the process of:
- Analysing knowledge that appears in various forms including forms adapted to illiterate patients (color codes, presence of photos).
- Act on knowledge: the patient reflects on lived experiences and identify changes to make in lifestyles.
- Work in interaction with other learners, which gives education a social dimension.
- Decide on the implementation of actions considering individual, cultural, social, economic context.
- Develop personal to-do-list ‘schedule’ reminders for actionable items including medical checkups, times for drugs and daily routine exercise. For instance, session on dietary control will have a strong focus on fat and may adopt the protocol of Food Sensations‱ ® for Adults [23]. These training days were implemented with 10 – 12 patients (1.5Hours) and the 4 sessions will take place over a period of two months starting from the inclusion of patients. We propose about 18 classes/session x4 sessions/lifestyle value x3 values’ constituting 216 training classes to occur in two months as first intervention, which will afterwards be repeated twice by peer-educators. Data collection will include pre-intervention and x3 post-interventions occurring at 3, 6 and 12 months.
Eligibility criteria
Inclusion criteria: T2D patients of ≥18 years of age followed in the diabetes units of the participating health facilities and doing regular consultations, T2D patients who accept to undergo the whole process of peer education, patients who agreed to perform all bio-clinical measures included in the protocol, and those with intercurrent illnesses who consent to lifestyle and physical activity monitoring were selected as eligible.
Exclusion criteria: Non-diabetic patients, patients with type 1 diabetes, patients with intercurrent illnesses who dissent to lifestyle and physical activity monitoring were excluded. Pregnant women and individuals with other illnesses such as arthritis and cancer are also excluded, except incidental T2D with intercurrent illness.
Interventions
T2D patients were randomly allocated in a 1:1 ratio to either a) intensive lifestyle education using network of T2D patients as peer-educators or b) usual lifestyle education. Learning and teaching activities were carried out with T2D patients to lead them on the journey of peer-education. Educational materials for the trial were printed and distributed.
Experimental: intensive lifestyle education using network of T2D patients as peer-educators
This intervention group will follow the whole process of peer education described above, in addition to the classical diabetes management consultations, which includes a counselling session, measure of blood glucose, blood pressure, weight and height, complete clinical examination, and a prescription or a renewal of diabetes treatment.
No Intervention: No Lifestyle counselling
The group subjected to educations will follow the whole process of peer education described above. The control group will perform these "classic" individual consultations but will not follow the whole process of peer education. The classical management in diabetes consultations consists of:
- A counselling session
- A measure of blood glucose
- A measurement of blood pressure
- A measure of weight and size
- A complete clinical examination
- A prescription or a renewal of treatment (diabetes pills, insulin, IEC, statins etc)
Strategies to improve adherence
To improve adherence to the intervention among the experimental group, intermittent phone calls were placed to the patients to remind them of partaking in the various scheduled training sessions. Also, the need to attending health facilities for regular routine check-up was stressed during the intervention sessions.
Further, adherence was buoyed by the relevant concomitant care, ensured by the blinding process. That is, healthcare providers in the various health facilities were aware of their patients participating in the trial, but unaware of those randomized into intervention group. Therefore, the healthcare providers were made to provide all participants with their usual service as concomitant care free-of-charge (i.e. at no cost to the participant) to be paid later by the research, which enhanced adherence.
Outcomes and Statistical Analysis
Outcomes
Primary outcome measure
The primary outcome measure at 12 months follow-up is the mean change in HbA1c from baseline following the interventions. The HbA1c was measured at baseline, 3, 6 and 12 months using the established standard diagnostic test method, according to the protocols of the health facilities. HbA1c was collected at each scheduled sessions with the patients in the experimental group - viz at baseline and subsequently after the lifestyle intervention at 3 months, 6 months, and 12 months. The samples were collected at fasting state at each schedule sessions, except where determined to be random. For the control group the test were done in the facility during their normal routine health checkup.
Secondary outcome measures: Changes in
Body weight: Secondary outcome measure is the mean weight change at 3, 6 and 12 months from baseline following interventions. Height, weight, and waist circumference of participants will be measured at baseline and at the proposed intervals. Height and weight of participants will be measured according to methods adopted in the various health facilities. This will be done with participants wearing light clothing and without footwear. Weight in kilograms divided by square of height in meters will be used to derive the body mass index (BMI). Waist circumference (WC) will be measured using a flexible measuring tape at the nearest 0.1 cm.
Blood pressure, glucose, and lipids profile : Blood glucose and lipids profile at 3, 6 and 12 months from baseline following interventions will be assessed. Plasma glucose, total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides will be measured by enzymatic colorimetry (Cardiochek Professional Analyser, Polymer Technology Systems, USA). Blood pressure will be measured by automatic oscillometry (Omron, Australia) from the non-dominant arm after 5 minutes rest with the mean of two measurements recorded.
Social support, diabetes stress, and quality of life : Measures of behaviour will be collected at baseline, 3, 6 and 12 month follow-up. The Food Frequency Questionnaire (Diet Questionnaire for Epidemiological Studies, DQES)[24], social support (Multidimentional Scale of Perceived Social Support, MSPSS)[25],depression and suicidal ideation (Patient Health Questionnaire 9, PHQ9) [25], and quality of life (WHOQoL questionnaire) [26]
Sample size
The required sample size to estimate the primary outcome at 12 months follow-up is 180 participants. A total number of 90 T2D participants per study arm was determined as required to detect an effect size of 0.23 assuming a within group standard deviation of 0.5 at 80% power and two-sided 95% significance level assuming an attrition rate of 15%.
Statistical Analysis The principle of intention-to-treat was followed in the full analysis set. Trial participants will be compared according to the group which they were allocated in the main analysis. We will not impute any missing data. The per-protocol analysis will comprise participants who complied with all the sessions.
Demographic characteristics: The baseline characteristics will be reported following the Consolidated Standards of Reporting Trials (CONSORT) guideline [27]. Means and SD or median and interquartile range will be reported as appropriate for continuous data, while categorical data will be reported as counts and percentages.
Primary glycaemia outcomes: Primary glycaemia outcome is average HbA1c (%) in each of the two randomised groups at 3-, 6- and 12-month follow-up. Mixed-effects regression analysis will be used to
assess difference between the two groups at the recorded timepoints. We will report the mean difference and two-sided 95% confidence interval (CI). However, if normality assumption is not satisfied, quantile regression will be utilised and median difference between the two groups and two-sided 95% CI will be reported.
Secondary outcomes: For secondary outcomes that are measured in interval scales, we will use the same approach specified for analysing the primary outcome. Secondary outcomes that are binary or categorical, we will use binomial, multinomial or ordinal logistic regression as appropriate to compare the two groups. Estimates of risk ratios or odds ratio as appropriate will be reported and their two-sided 95% CI.
CONCLUDING STATEMENT OF SIGNIFICANCE
National estimates shows that T2D, obesity, cardiometabolic syndrome and related risk factor are on the rise in Nigeria [11, 28, 29], with significant burden on the health systems. Evidence shows that education forms the framework on which medication, nutrition, and other lifestyle modifications are built to improve outcomes of patients with T2D [30]. Therefore, this trial will contribute to the evidence on T2D patients-led intensive peer education, given limited evidence on the effectiveness of peer education in the management of T2D within the low-income setting such as Nigeria. This is pertinent given the lack of robust translational research within the context of diabetes prevention in Nigeria. Hence, the results of this trial will provide the best evidence to guide diabetes management in Nigeria. Outcomes of this research will be disseminated to participants, their communities, and all stakeholders and by publication.