IMC STAINING FOR PARAFFIN SECTIONS
Klaus H. Kaestner Lab, Suzanne Shapira
Abstract
Imaging mass cytometry (IMC) is a multiplexed imaging method that allows samples to be probed with up to forty antibodies at one time. This enables visualization of multiple cell types simultaneously in the native tissue microenvironment. For example, IMC can be applied to study the interactions between endocrine cells and the immune system in pancreata from donors diagnosed with type 1 diabetes. This protocol describes a technique for IMC staining of paraffin sections with a defined antibody panel to identify immune, islet, endocrine, and stromal cell types of the pancreas.
Steps
Procedure
Use pressure cooker or heat block to preheat Antigen Retrieval Solution to 95°C before starting.
Bake slides in 56°C over for 20 min.
Dewax slides in xylene in the fume hood for 5 min X 2.
Hydrate slides in descending grades of ethanol (100%, 95%, 80%, 70%), 5 min each.
While you are dewaxing and hydrating slides, prepare 50 mL 1x PBS by diluting 10x PBS with Milli-Q water.
Rinse slides in PBS for 5 min.
Insert slides with tissues into preheated Retrieval Solution and incubate for 30 min at 95°C .
Following incubation, remove from pressure cooker or heat block and place the tube containing the retrieval solution and slides on a lab bench and cool to room temperature, approximately 30 min (or until it reaches room temperature).
Wash the slide with PBS for 10 min.
Use PAP pen to encircle sample.
Block with 3% BSA in PBS for 45 mins at RT.
To prepare the antibody cocktail, calculate total volume of antibodies (at concentrations specific for the assay) and bring up to final volume with 0.5%BSA in PBS.
Incubate overnight with antibody cocktail at 4°C in hydration chamber (We use a slide box where the slides rest on the shelf and the bottom is covered by wet paper towel).
Wash in PBS for 8 min x 2.
Stain the tissue with Ir-Intercalator (1:400) in DPBS for 30 min at RT.
Rinse in ddH²O for 5 min.
Air dry the slide for at least 20 minutes at RT.
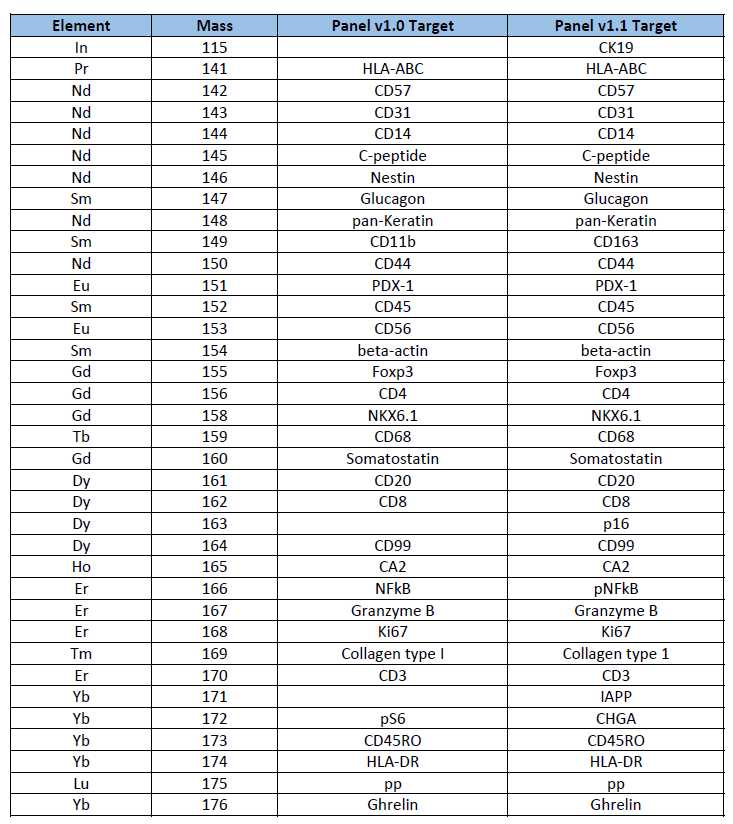
List of IMC panel
1. Panels used currently
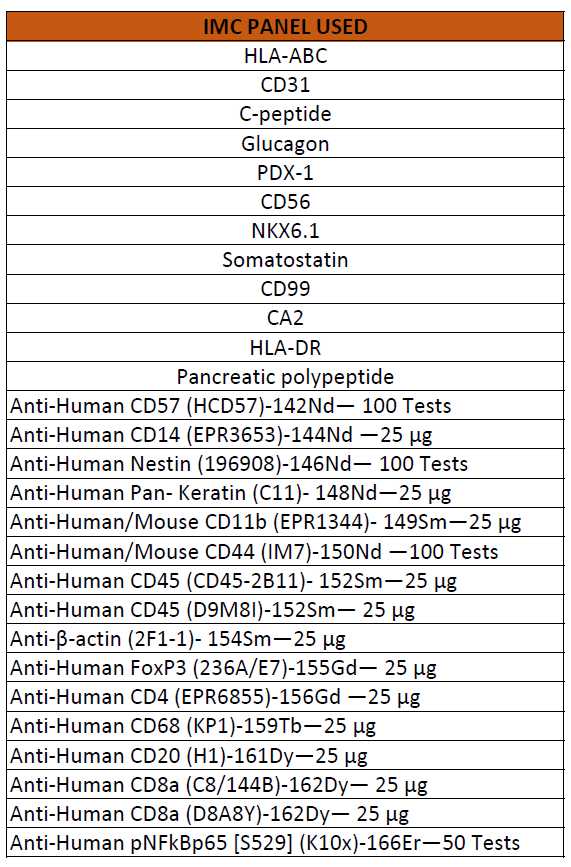
2. Panel combinations/versions used so far