Human ovarian tissue procurement and processing for research
hannah.anvari, asma.giornazi, shriya.shah, maryellen.pavone, francesca.e.duncan duncan
Abstract
Purpose: This protocol is intended for use in the collection and storage of human ovarian tissue n a research setting. The protocol details the collection, processing, and long-term storage of human ovarian tissue as formalin-fixed paraffin-embedded (FFPE) samples or frozen samples.
Steps
Collection of ovarian tissue for research (in pathology)
Ovaries are evaluated grossly per standard protocol in Northwestern Medicine Pathology. Ovaries are measured, external surface examined and evaluated for neoplasia.
If no significant gross pathology is found, provide any ovary sections to the U54 research coordinator that are not required for clinical diagnosis.
If gross lesions are found, provide non-lesioned tissue, if possible, based on individual gross assessment. The sign-out pathologist or study pathologist should be consulted in case of questions on what is appropriate to submit or retain.
Human ovarian tissue processing (in lab)
Ovarian tissue samples are collected from Northwestern Pathology department and placed on ice immediately following collection. Research coordinator will bring research specimens to the lab on ice (2-4 °C). Samples should be transported in blue, labeled biohazard cooler bag (Fig. 2A, B).

Tissue should be kept at 2-4°C throughout all processing steps and should be processed within 24 hours of procurement. Tissue is kept in ORIGIO Handling media throughout procurement and processing (Fig. 2C). Once in the lab, tissue is processed at room temperature (RT).
On arrival, tissue should be measured for length, width, and thickness (Fig. 2D). Also note the approximate anatomy of cortex (black) to medulla (white) (Fig. 2E). The cortex should be on the outermost edge surrounding the entire tissue piece and will have a glossy, somewhat translucent appearance. The medulla should be the innermost portion if the ovarian tissue slice is lying flat and will likely contain fibrotic looking, white tissue especially in postmenopausal ovaries. Prepare a 60 mm dish with 3-5 mL ORIGIO Handling media. The tissue should be submerged, but not floating in media.
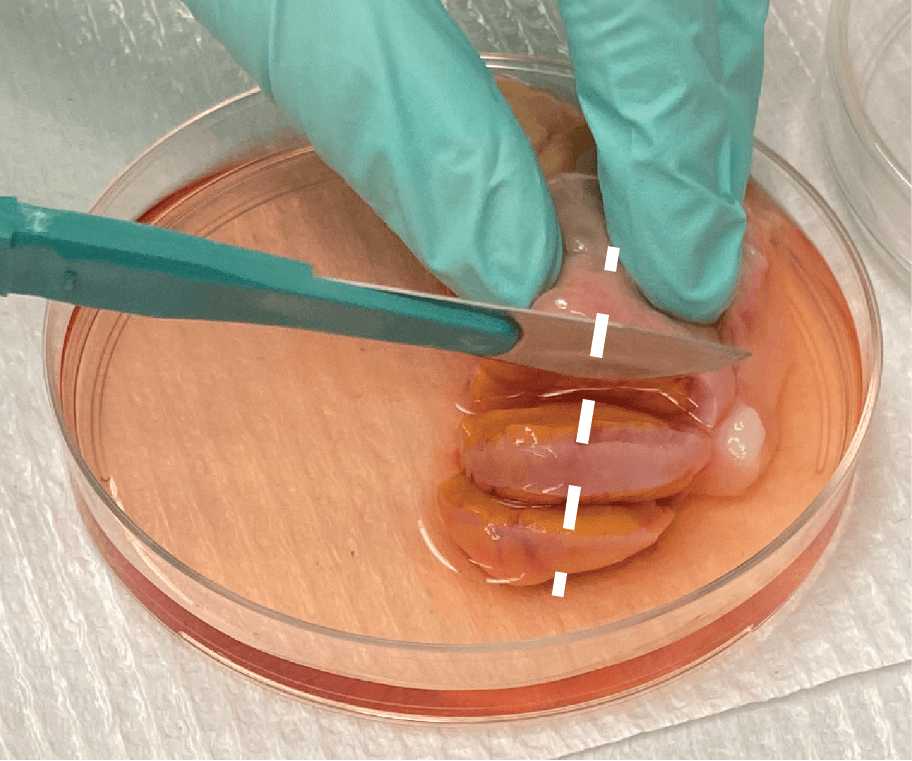
Ovarian tissue is bisected laterally using a No. 10 or No. 22 scalpel, depending on the size and thickness of tissue (if thicker or longer, use a No. 22 so that cuts are more even), to give two halves of ovarian tissue (Fig. 2F).
One half should be divided into 20-30mg pieces that include both cortex and medulla tissue (Fig. 2G).
Pieces to be frozen are weighed (Fig. 3I), washed in PBS solution, and then placed in an individual 1.5 mL micro centrifuge tube with minimal PBS remaining on the tissue. Tubes can then be placed on dry ice for 15 min before being moved to -80°C freezer for long-term storage (Fig. 3J).
Shipping fixed (FFPE) human ovarian tissue
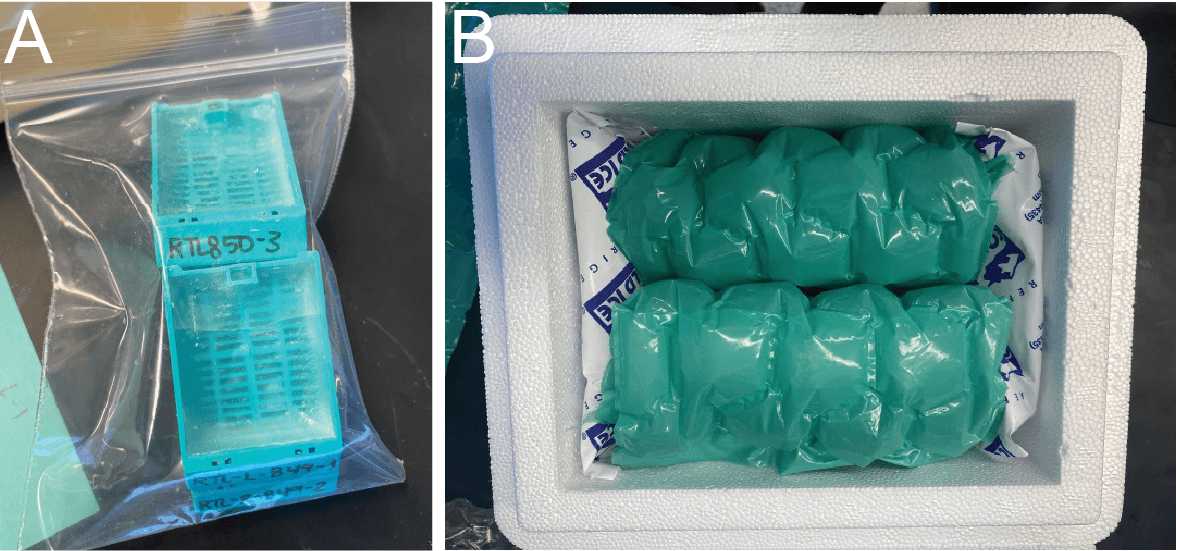
All fixed tissue samples are in embedded blocks that can be shipped at RT or with ice packs (warmer months).
Multiple blocks can be stacked for safe shipment (Fig. 3A) and then placed a box surrounded by padding for transport (Fig. 3B).
Shipping frozen human ovarian tissue
All frozen samples should rest for at least 24 hours after initial processing/freezing to ensure stability during shipment.
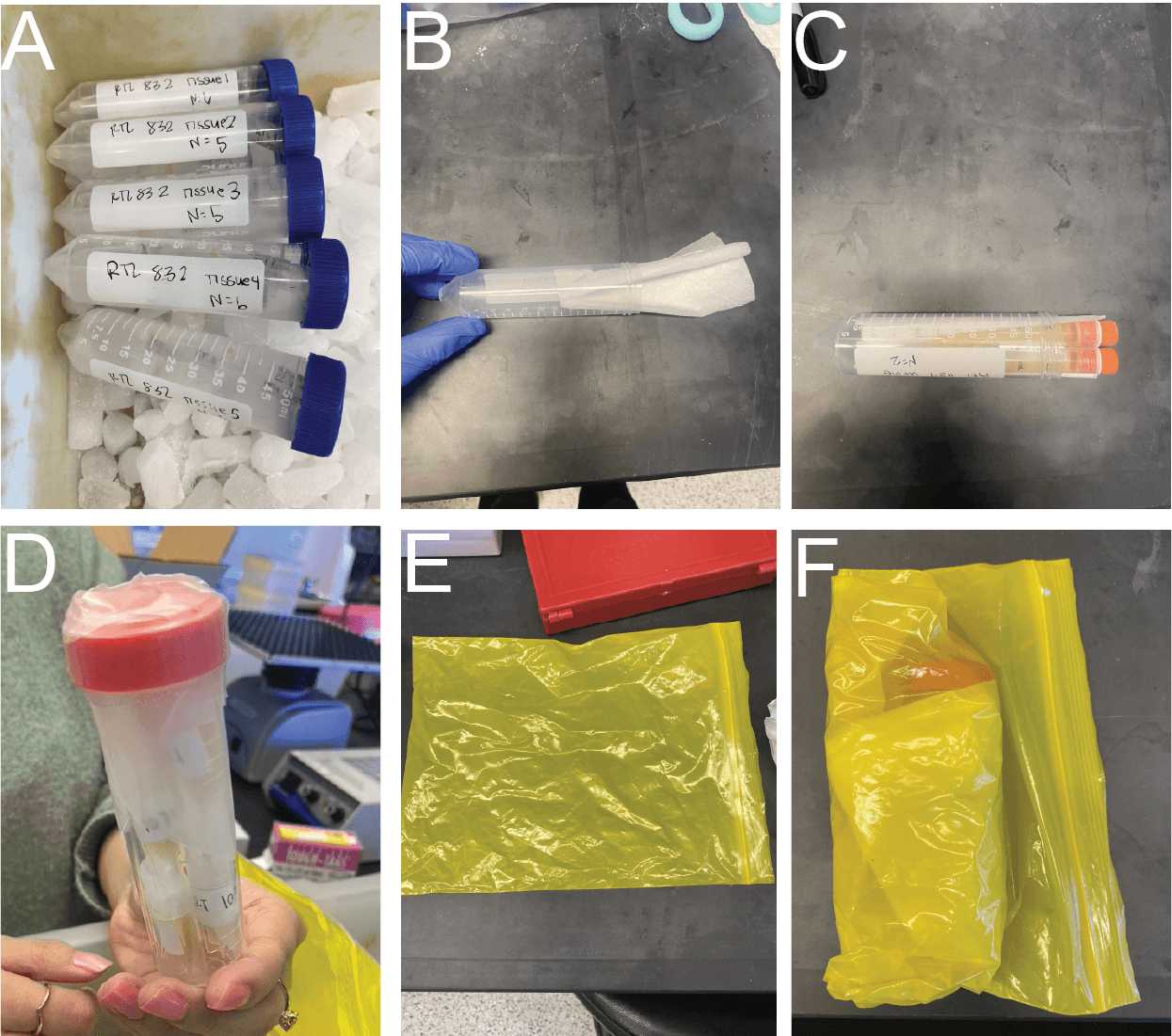
Samples are removed from -80°C storage into a dry ice holding bucket while being sorted into shipping boxes (Fig. 4A).
All frozen vials should be added to a larger secondary 50 mL conical tube lined with a Kimwipe (Fig. 4B-C). Secondary containers need to be wrapped with parafilm (Fig. 4D)
Once all samples are ready in secondary containers, they should be placed in a sealable bag (Fig. 4E) with additional Kimwipes to absorb any fluid that could spill during transit (Fig. 4F).
The bag should be completely submerged in dry ice (over, under, surrounding the sides) for safe shipment. Lab members will fill out appropriate documentation to ship with dry ice.