Human Pregnant Fallopian Tube Tissue Collection and Preservation Methods - UCSD Female Reproductive TMC
Scott Lindsay-Hewett, Valentina Stanley, Louise Laurent, Mana Parast
Abstract
Human pregnant fallopian tube tissue collection and storage protocol for HuBMAP's UCSD Female Reproductive TMC.
Steps
Preparation
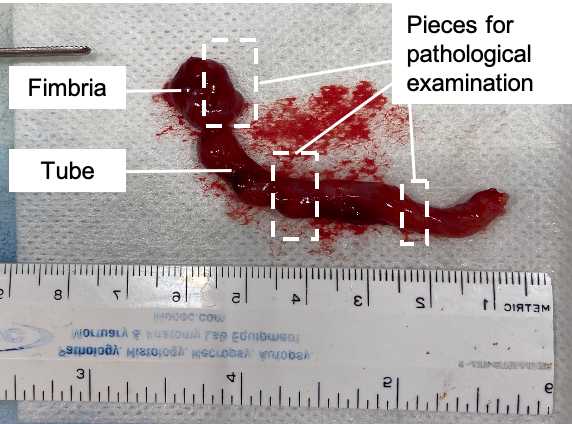
Fallopian tubes should be collected into empty sterile cups right after sterilization (salpingectomy or tubal ligation). Keep left and right tubes separate.
Wash the tissues in cold PBS while maintaining the original orientation.
Preserving the fimbria
Separate the fimbriated end from the tube (if not already separated).
Divide the fimbriated end lengthwise into 2 or 3 equal sized sections (depending on size).
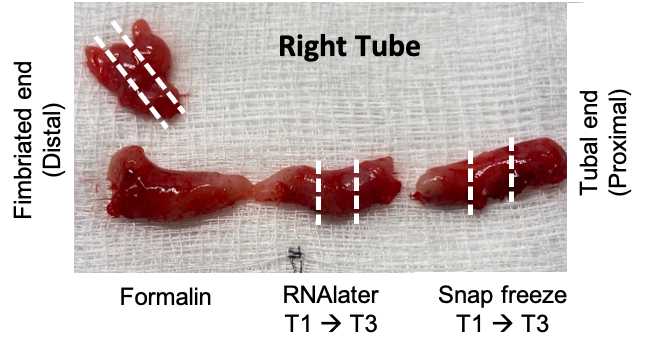
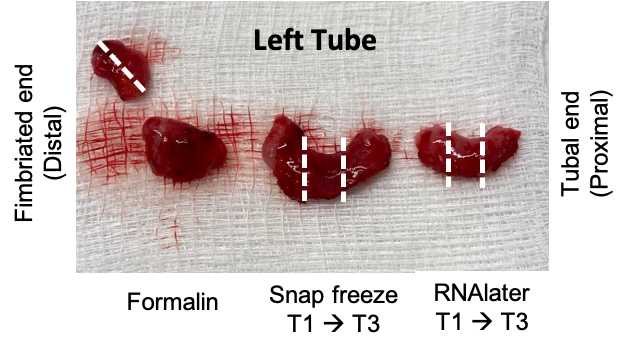
Place one fimbriated piece into a 10% formalin-filled tube (1st priority), the other into RNAlater (2ndpriority), and the third to be snap-frozen (3rdpriority).
Preserving the tube
Collect cross sections of the tube into 10% formalin (x1), RNAlater (x3) and to be snap-frozen (x3). Each piece should be about 5mm in length, depending on the total length of the tube. If there is an excess of tissue, the formalin piece(s) can be larger.
Collect the formalin-preserved piece from the most distal end (closest to the fimbria).
Flash-freezing
Drop the snap frozen labeled tubes into liquid Nitrogen. Leave for ~2-10 minutes, then store in -80C freezer.
Processing with RNAlater
Place RNAlater tubes into a 4C fridge. Let these sit for 24-48 hours, then remove the RNAlater with a sterile transfer pipette and store in a -80C freezer.
Formalin fixing
After one week in formalin, place each formalin-fixed piece into a labeled cassette to be processed into FFPE blocks.