High resolution respirometry of isolated mitochondria from adult Octopus maya (Class: Cephalopoda) systemic heart
Ana Karen Meza-Buendia, Omar Emiliano Aparicio-Trejo, Fernando Díaz, Claudia Caamal-Monsreal, José Pedraza-Chaverri, Carolina Álvarez-Delgado, Kurt Paschke, Carlos Rosas
Isolation of mitochondria
Respirometry
Adult octopus
Isolation of mitochondria
Octopus maya
Octopus
High resolution respirometry
heart
respiratory states
Abstract
Mitochondrial respirometry is key to understand how environmental factors model energetic cellular process. In the case of ectotherms, thermal tolerance has been hypothesized to be intimately linked with mitochondria capability to produce enough adenosine triphosphate (ATP) to respond to the energetic demands of animals in high temperatures. In a recent study made in Octopus maya was proposed the hypothesis postulating that high temperatures could restrain female reproduction due to the limited capacity of the animals’ heart to sustain oxygen flow to the body, affecting in this manner energy production in the rest of the organs, including the ovarium (Meza-Buendia et al. 2021). Unfortunately, until now, no reports have shown temperature effects and other environmental variables on cephalopod mitochondria activity because of the lack of a method to evaluate mitochondrial respiratory parameters in those species’ groups. In this sense and for the first time, this study developed a method to obtain mitochondrial respirometry data of adult Octopus maya ’s heart. This protocol illustrates a step-by-step procedure to get high yield and functional mitochondria of cephalopod heart and procedure for determining the corresponding respiratory parameters. The procedure described in this paper takes approximately 3 to 4 hours from isolation of intact mitochondria to measurement of mitochondrial oxygen consumption.
Before start
Pre-chill glassware before starting the procedure.
Steps
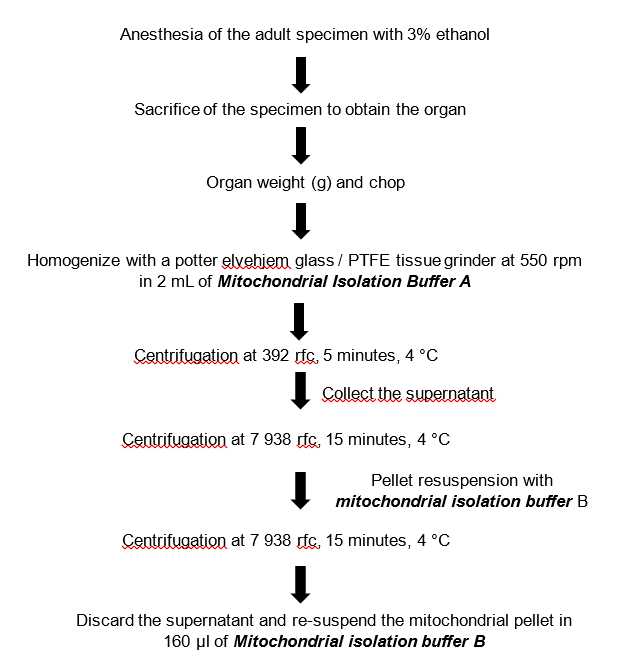
Isolation of mitochondria from the systemic heart of adult octopus
Starve octopus before the isolation experiment.
Sacrifice an adult Octopus maya specimen (about 1kg) previously anesthetized with 3% alcohol and quickly remove the systemic heart from the mantle cavity.
Place the systemic heart immediately on a Petri dish On ice and add 1mL of mitochondrial Isolation Buffer A to rinse the organ.
Cut the systemic heart into pieces with scissors and mince into smaller pieces with a scalpel, which should be done while the Petri dish is On ice.
Transfer the cut pieces of the organ to a homogenization tube with 2mL of cold mitochondrial isolation buffer A.
Homogenize the systemic heart using Potter-Elvehjem PTFE pestle and glass tube (Sigma-Aldrich P7859-1EA) homogenizer operated by a drill at 500rpm,0h 0m 0s.
Three to four stocks are made to homogenize the previously minced tissue.
Homogenization is done in a container with ice and the ice homogenization tube must not be removed.
Transfer the homogenate by decantation to a pre-cooled 2 ml Eppendorf tube® and centrifuge at 392rcf,0h 0m 0s at 4°C for 0h 5m 0s.
Transfer the supernatant obtained from the previous step to another pre-cooled 2 ml Eppendorf tube® with a micropipette and keep On ice.
Centrifuge the transferred supernatant at 7938rcf,0h 0m 0s for 0h 15m 0s at 4°C ('mitochondrial pellet formation').
Discard the supernatant by decantation and wash off the pellet.
First add 1mL of cold mitochondrial isolation buffer B and re-suspend the pellet gently with a soft bristle brush (natural bristles).
Re-suspend the pellet, add 1mL of cold mitochondrial isolation buffer B. Subsequently shake gently and quickly to homogenize and keep On ice.
Centrifuge at 7938rcf,0h 0m 0s at 4°C for 0h 15m 0s.
Discard the supernatant by decantation and conserve the pellet.
Add 160µL of cold mitochondrial isolation buffer to concentrate the sample and resuspend the pellet in the same way as in steps 10 and 11.
Measure mitochondrial concentration using the Bradford method (Bradford 1976). According to our own experimental results, mitochondrial suspensions from the systemic heart of Octopus maya adults contain ~14mg protein/ml per 1g of minced tissue. Mitochondria are now ready to be used in experiments of respirometry. Use the preparation within 1h 0m 0s–4h 0m 0s for better functional responses.
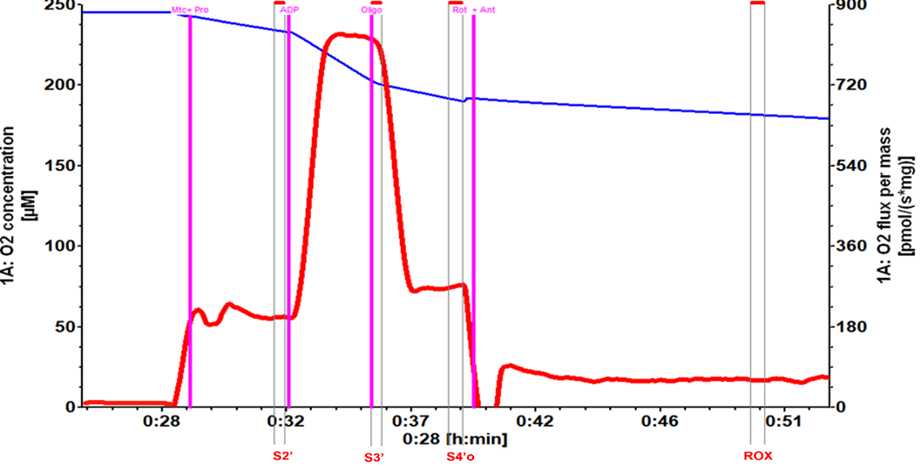
Measuring mitochondrial respiration: High-resolution respirometry (HRR)
24°C). Equipment setup: Calibration of polarographic oxygen sensors
Add 2mL of mitochondrial respiration buffer (MiR05) to the chamber (this protocol was developed using a 2mL volume), and the O₂ sensors are calibrated.
Wait for an equilibrium with atmospheric oxygen and the required experimental temperature.
The system reaches the steady basal consumption state of the system in operation, a point where the O₂ consumption rate is constant.
Start recording of oxygen consumption.
Verify that the recording is stable and that no drifts are apparent.
Substrate/inhibitor titration (SUIT) analysis
Table 1. Action and concentration of agents used for measuring mitochondrial respiration of isolated mitochondria from the systemic heart of Octopus maya. Octopus maya .
| A | B | C |
|---|---|---|
| Reagent | Action | Final concentration |
| Proline | Amino acid substrate | 5 mM |
| ADP | Substrate for the generation of ATP | 1.25 mM |
| Antimycin | Complex III inhibitor | 12.5 µM |
| Rotenone | Complex I inhibitor | 2.5 µM |
| Oligomycin | ATP synthase inhibitor | 2.5 µM |
Use an appropriate Hamilton microsyringe (Oroboros Instrument), add mitochondria (Mtc) to obtain a final concentration between 300μg/ml-500μg/ml .
This step is followed by a rapid and transient decrease in oxygen content of the chamber followed by a slower decrease caused by respiration of the mitochondria, commonly referred to as Respiratory State 1.
Use a Hamilton microsyringe (Oroboros Instruments), add Proline (Pro) to a final concentration of 5millimolar (mM).
Observe a faster rate of oxygen consumption because of basal activity of the respiratory chain to counteract proton leakage from the inner mitochondrial membrane, which represents Respiratory State 2’ (S2’).
Record for ~ 0h 2m 0s.
Add 5µL ADP (500millimolar (mM) ADP stock solution) to obtain a final concentration of 1.25millimolar (mM).
A faster oxygen consumption is observed and represents Respiratory State 3’ (S3’), where ATP production is the principal contribution of oxygen consumption.
Record until the rate of oxygen consumption begins to drop.
Add 1µL of oligomycin A (5millimolar (mM) oligomycin stock solution) to obtain a final concentration of 2.5micromolar (µM) and induce Respiratory State 4’ (S4’o). With this procedure OXPHOS is inhibited by oligomycin and the rate of oxygen consumption begins to rapidly plateau (steady state).
Record for ~ 0h 2m 0s.
Add 2.5micromolar (µM) rotenone (Rot) plus 12.5micromolar (µM) antimycin A (Ant) to obtain residual or non-mitochondrial respiration (ROX).
Both compounds inhibit the electron transport system flux and induce a rapid decrease in oxygen consumption rate until it remains constant.
Record for ~ 0h 5m 0s and then stop recording.
The Respiratory States S3’ and S4’o, were corrected for the respiratory state ROX (residual non-mitochondrial respiration): S3= S3’-ROX and S4= S4’o-ROX. The respiratory control parameter (RC) was defined as S3/S4o, while respiration directly attributable to OXPHOS was defined as S3-S4o, which is the phosphorylation state parameter (P). See Fig 2.