Handling and Sampling Small Non-Volant Mammals - ISL Peru
Gideon Erkenswick, Mrinalini Watsa, Cristian Tirapelle, Pamela Sánchez-Vendizú, Leticia Gutiérrez, Daniel Llancachahua-Tarqui
field anaesthesia
biological sampling
insitulabs
isoflurane
small mammals
wildlife handling
wild rodents
Disclaimer
This protocol is actively used by Field Projects International at the Estación Biológico Los Amigos, Madre de Dios, Peru. It is revised annually to reflect improved capture, handling, marking, and sampling methodology. It has been reviewed by the ethics committees of multiple institutions. No author nor affiliated institution takes responsibility or bears any liability for the use of this protocol by others. The protocol is listed as having sensitive content since it involves biosampling from wildlife. Note: these procedures should be carried out only by trained personnel, and are not recommended for use without first obtaining all required permissions.
Abstract
Program Timing
Sample collection occurs annually during the rainforest dry season (June - August). Sample transport and analyses occur between September and April.
Team Composition
This protocol is intended to be carried out by a team of 4 individuals, including at least 2 trained personnel. Roles include: (1) designated handler; (2) chemical restraint operator; (3) sampling assistant; (4) data recorder.
Program Overview
500-meter long transects of Sherman and Tomahawk live traps are set up at multiple locations encompassing major habitat types surrounding the field station (terra firme, flood plain, swamp, bamboo, successional forest, primary forest, edge forest, etc). Captures take place over 3 consecutive days at each location throughout the sample collection period. Dependent on resource availability and personnel, we strive for an annual sample size of 300 subjects from EBLA.
Capture Overview
Trap transects are baited and activated at 17:00 hours and revisited at 05:00 hours the following day to check for successful captures -> unsuccessful traps are deactivated, successful traps are collected and brought to a nearby tent for processing (15 - 30 min, depending on the number of captures) -> one-by-one, animals are extracted from the traps, chemically restrained for safe processing of morphometric measurements, photographs, nonlethal tissue collection (skin biopsy, fur/nail, blood, mucosal swabs), and placement of microchips or ID tags (10-15 min) -> after processing animals are transferred into a light and breathable cloth bag or covered holding cage, with 3-minute checks for breathing and movement until fully alert (on average ~ 5 - 10 minutes) -> once alert, animals are immediately released at the same site of capture along the transect.
Capture (Additional Details)
Animals are diverse in their behaviors, attraction to bait, and use of forest habitat. The bulk of captures will be achieved via baited terrestrial sherman traps, however, some traps will be fastened higher up, onto tree substrates, to increase the likelihood of sampling species that forage in trees, or spend little time on the ground.
Before start
All material needed to process the animals should be double-checked against the MATERIAL list, and packed the day before. Only the hot water thermos and ice packs for the cooler are collected in the morning of processing.
A processing tent should have been already set from the previous night, next to the transect.
Steps
ROLES
Team Composition
This protocol is intended to be carried out by a team of 4 individuals, including at least 2 trained personnel. Roles include: (1) designated handler; (2) chemical restraint operator; (3) sampling assistant; (4) data recorder.
Designated Handler
This is a trained and senior researcher/veterinarian that is experienced with every step of the small animal capture process. They are responsible for animal sherman trap removal, as well as direct handling during the processing phase, and release.
Chemical Restraint Operator (CRP)
This is a trained and senior researcher/veterinarian that has experience with administering small animal anesthesia. Isoflurane is the primary and intended anesethetic, but this person should be prepared to use an alternative protocol as may be necessary. This person is also responsible for monitoring animal vitals and determining when processing should conclude, even if full sampling has not been achieved.
Data Recorder
Will perform data recording on the animal processing sheet MammalFormCris22_hardcopy.pdf
- Write down the weight of the animal and any other measurements, sample codes, and important time stamps.
- Recording notes from the handler
- Storing samples in the dedicated sample boxes
- Notify the vet team when it is time to check the vitals (every 3 minutes from the previous reading) and make sure that all the vitals are taken at each check
- Notify the team at 10 minutes from the beginning of the processing of a given animal. By this time, processing should be done, but if not, the team has approx. 5 minutes to finish
| A | B | C | D |
|---|---|---|---|
| Discovery (real time, 24hr): | Out of trap (SW): | Recovery (SW): | Release (SW): |
Example from the mammal processing form showing important time stamps
| A | B | C | D | E | F | G | H | I | J | K | L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | |||||||||||
| Resp Rate | |||||||||||
| PalpebralRef | Y N | Y N | Y N | Y N | Y N | Y N | Y N | Y N | Y N | Y N | Y N |
| Tail tone | Y N | Y N | Y N | Y N | Y N | Y N | Y N | Y N | Y N | Y N | Y N |
Example from the mammal processing form showing relevant parameters that are recorded for anaesthesia monitoring.
Sampling Assistant
Responsible for:
- Taking pictures of the animal.
- Assisting the HANDLER by passing tools, designated tubes or bags to collect each sample.
- Storing samples according to the protocol in use.
- Help the DATA RECORDER to double-check that all samples have been recorded on the processing form, and that the sample codes on the tube match those written on the form.
TRAP INSPECTION
The morning of processing, make sure the hot water thermos is filled and place an ice pack inside the cooler bag.
The team leaves the station at 05:30 hours, during sunrise, with all materials needed to process animals.
Three team members are involved during trap inspection, the fourth person prepares the processing tent. During trap inspection, two people move along the transect checking each Sherman trap and calling out information to the third person, who is completing the "Small Mammal Transect Form" using the ODK COLLECT app.
If a trap is empty , the bait must be poured out and trap deactivated. LEAVING A TRAP OPEN COULD RESULT IN AN UNTENDED CAPTURE AND INJURY OR DEATH TO AN ANIMAL
If an animal is captured , the trap + animal inside is brought to the processing tent. Make sure the trap is marked (using masking tape and a marker) with the code and indicating its specific location.
When the team returns from trap inspection, all Sherman traps should be placed outside the tent, in shade, and protected from rain
PROCESSING SETUP
Setup the processing table:
- clean table surfaces (in order) with 10% diluted bleach -> water -> 70% ETOH,
- take out enough sampling supplies for the first animal and set on table or in secondary animal tray,
- take out measuring materials,
- ready the tool sterilization kit,
- ensure space for primary animal tray,
- check that camera is ready with lots of free space on card.
Before beginning, start two STOP WATCHES and the VOICE RECORDER. Say aloud: (1) the full date and time; (2) the weather and temperature; (3) the number of animals captured; (4) the transect site; (5) the team members participating. The devices should run continuously until the end of the entire capture session. Important time stamps should refer to the stopwatch time.
Setup the Somnoflo isoflurane vaporizer:
Connect Somnoflo to the power-bank, and to the isoflurane bottle using the specific connector.
Select the appropriate size nose-cone.
Make sure the anaesthetic chamber and the nose-cone are attached to their respective inspiration and expiration tube lines, the latter are connected to the charcoal filter, which sits on a dedicated stand that allows filtered air to pass through the bottom.
Adjust the tube line clips so that flow is initially directed toward the chamber.

PROCESSING
EXTRACT ANIMAL FROM TRAP
Bring Sherman trap inside the the tent.
Transfer animal from the Sherman trap into a cotton bag by tipping the trap upside-down with the door opened inside the cotton bag. IF A RECAPTURE IS SUSPECTED, check the animal's ear tag number with their head facing a corner of the bag.
- If RECAPTURE from the current season, do not proceed. Instead, at a convenient moment, bring animal back to the trapping site and release.
- If not, proceed as usual
Empty the bag that contains the animal directly into the anasthetic chamber.
ALTERNATIVE: Release animal from Sherman trap into a deep bucket -> pick up animal (using protective gloves) and placed inside anaesthetic chamber -> quickly slide chamber door closed.
Once inside chamber, SELECT "high flow" on the touch screen and start the induction at 4% isoflurane with flow rate preset to 500 ml/min.
CHEMICAL RESTRAINT OPERATOR will monitor closely the behaviour of the animal while in the chamber. Watching carefully how the animal is breathing and for general signs of induction. Lowering the head or assuming a lateral recumbency is generally a sign that the animal has reached a sufficient plane of anaesthesia.
Quickly remove the animal from the anaesthetic chamber, and:
Record ANIMAL WEIGHT on the digital scale, then place animal onto the processing tray with its face positioned inside the nose cone.
Select low-flow setting (preset at 1.8% Isoflurane at 200ml/min). May need to adjust concentration to 2% fairly quickly, if animal appears to be waking up.
Consider adjusting the flow setting according to the weight of the animal following the chart supplied by Kent scientific. It is also possible to adjust the percentage of isoflurane.
The CHEMICAL RESTRAINT OPERATOR is in charge of monitoring anaesthesia throughout animal processing. Monitoring is mostly performed by checking the respiratory rate and the breathing pattern, the palpebral reflex and the tone of the tail. A trained operator may increase or decrease the percentage of isoflurane delivered according to the depth of anaesthesia according to how the animal responds to stimuli and depending on the procedure to be performed.
Tracking & Life Tags
Prior to release a subset of animals will be fitted with a UHF tracking devices (Cellular Tracking Technologies). Tags are temporarily glued to the dorsum (duration ~ 20days). Animals will only be considered for a tracking tag if their weight is >= 60g.
Transmitter attachment:
- Transmitters are light weight (3g or 6g) and will be applied to animals that pass the 5% cutoff in weight only.
- Transmitters should be attached either by glue or by harness:
- Glue attachment: Transmitters should be attached to the area between the shoulder blades. For animals with shorter hair, transmitters will be attached without clipping hair, otherwise clip hair down close to the skin before adhesion (do not completely remove hair). Apply a very thin layer of adhesive (Perma-type surgical cement) on the transmitter and on the animal. Let it stand for 5 mins until the glue bubbles. Then press the transmitter to the site applying a steady pressure for five minutes. To be certain the tag is fully fixed, do not release the animal immediately, but hold for 10 more minutes, at a minimum. Glued tags will naturally drop off in under one month
- Harness attachment (NOT YET VALIDATED): Using stretch magic (0.7mm), we can build a loop system similar to that of the leg-loop system in birds for attachment of the tag between the scapulae of the animal. This material will last up to two months at the outer range, and then drop off, and will be used only on the largest of these animals (100g+).
Permanent identification:
- Additionally, we intend that all small-mammals will eventually receive a P-chip identification tag (https://p-chip.com/) which has been successfully used by SDZWA on the Pacific Pocket Mouse.
- P-Chips are micro transponder tags (500 μm x 500 μm x 100 μm) with photocells that are powered by a handheld laser wand connected to a computer or tablet and read through PharmaSeq’s p-Chip Reader software to emit a unique 9-digit signal (PharmaSeq Inc., Princeton, NJ).
- P-Chips are small, lightweight, and provide individual identification
- Tags come in an individually packaged sterile injector and are inserted subdermally at the base of the tail such that they are visible under the skin as a tiny black dot.
- Each p-Chip should be scanned with the handheld laser after tagging to record tag number
SAMPLING, PICTURES & MEASUREMENTS
The experienced HANDLER and the SAMPLING ASSISTANT are in charge of collecting samples and taking measurements. The recommended order of operations is:
DORSAL PICTURE
Take picture of fur on the dorsum
FACE PICTURE
Take pictures of the face and ventrum, quickly before the animal awakes.
FECES
Faecal samples are collected either from a surface where they have been dropped, or directly from the opening of the anus. Feces are often found in the Sherman trap and sometimes come directly during processing. Use designated, pre-labeled bags or tubes for feces
CUT/SHAVED HAIR
With a fine scissor, cut HAIR from the flank or dorsum of the animal and place it in a H-merc bag. Hold scissors parallel to the body to avoid cutting skin by accident. The body location from which the hair sample is collected should be reported in the processing sheet.
MEASUREMENT
Measure ears and the tail using a plastic ruler
PLUCKED HAIR
If the animal is not reacting to the above procedures, continue by plucking HAIR for DNA studies. it is important to include the hair follicle. To achieve this, a small amount of hair is grabbed with a pair of tweezers and plucked from the animal's skin. Transfer the hair into a pre-labelled, clean zip-lock bag marked as H-DNA followed by a serial number.
BIOPSY
If the animal is still not reacting, and IF THIS IS A FIRST-TIME ANIMAL CAPTURE, a 3mm long and 2mm high skin sample from the tip of the left ear pinna is collected:
- use an antiseptic wipe to disinfect the tip of the ear pinna, usually the left one.
- use a fine cutting scissor to cut a 3mm long and 3mm high biopsy from the tip of the ear pinna.
- any small amount of blood that may come from the biopsy can be collected with a microhaematocrit capillary tube by touching the blood drop to an open end of the tube.
ALTERNATIVE: a piece of sterile card stock may be placed behind the biopsy site, and then a sterile 3mm hole punch is pressed firmly on the site to quickly extract a skin biopsy
INDIVIDUAL IDENTIFICATION: EAR TAG PLACEMENT
Using the designated tagging tool, place the ear tag at the base of the right ear pinna.
BLOOD
To collect blood, first attempt the ventral tail artery :
- Position the animal so that venipuncture may be attempted at a 90 degree angle in the proximal 1/4th of the ventral tail. The artery is deep and right next to the tail vertebrae.
- Be ready to touch the blood drop that appears with the open end of a capillary tube
- Wait until the capillary tube is 3/4 full, then close both ends with clay.
- If the size of the animal allows it, collect a second capillary tube and from this make 2 blood smears (described in separate protocol), then break the capillary into a 1.7 mL tube with lysis buffer.
- Close the tube and shake it to homogenize the sample.
Also a good option, collect blood using the saphenous vein :
- Move the animal on its flank, hold the back leg facing up with two fingers while gently putting pressure on the femoral vein. By doing this the saphenous vein should engorge.
- Disinfect the lateral aspect of the calf with an antiseptic wipe.
- With the help of a cotton bud place a thin layer of vaseline to create an hydrophobic environment
- Move the fur to visualize the saphenous vein close to the knee area, and puncture the vein at a 45 degree angle with a 25 gauge needle, and follow same steps as above.
PICTURES
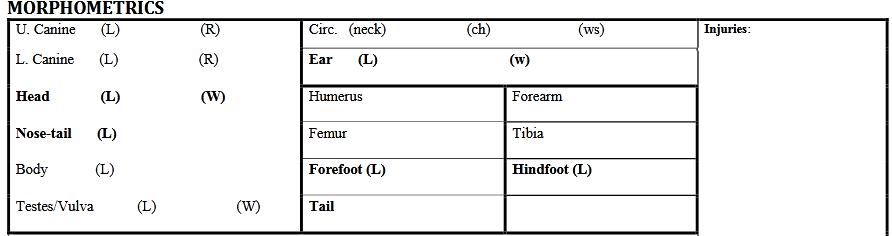
Now that most samples have been collected, it is possible to measure and take pictures of all the body parts highlighted in bold in the PROCESSING SHEET. For this, the animal can be re-positioned on sternal recumbency.
BUCCAL
Set the flow rate to OFF -> remove animal from the nose cone -> take 2 buccal swabs:
- Remove swab from its envelope in a sterile way; by pulling apart the bottom and top layers of the envelop, starting from the side opposite to the foam tip.
- Hold the swab from the stick and be careful not to touch any nontarget surface with the tip.
- Insert the tip to the buccal side of the mouth and rotate 4-5 times against the mucous membrane on the internal aspect of the cheek and on the gums. It is also possible to position the swab between the tongue and the gums.
- Place the swab inside the dedicated 1.7mL tube containing lysis buffer
- Break the swab in a sterile way, close the lid of the tube
POST-SAMPLING
Place animal inside a cotton bag, leaving the skin of the dorsum exposed.
Strongly suggest injecting subcutaneous fluids at this moment to replace the volume of blood extracted and counteract potential dehydration from time in the trap.
Close the animal inside the bag (if necessary place bag in a warm environment) during recovery from anaesthesia.
RELEASE
RELEASE
The animal should be brought back to the spot where it was captured for release.
The RELEASE TIME should be reported on the PROCESSING SHEET as well as any relevant behavioral observations at the time of release (i.e. immediate or slow release)
Score the quality of the induction, maintenance, recovery, muscle relaxation achieved with the anaesthetic protocol, and give an overall judgement on it. These information can be recorded on the PROCESSING SHEET.
CLEANING
- Clean the surfaces with 10% bleach -> then 70% alcohol
- Clean the anaesthetic chamber and the nose cone with 10% bleach -> distilled water -> then 70% alcohol.
- Disinfect resuseable materials with the dedicated sterilization kit: 10% bleach for 3 minutes -> distilled water rinse -> distilled water rinse -> 70% alcohol rinse.

Sterilization kit prepared with a rack and four 50ml falcon tubes. Courtesy of Thomas Parsons.
Once everything is set and ready, it is possible to proceed with the next animal.
END SESSION
FIELD WRAP UP
Once all animals have been released the team should:
- End the VOICE RECORDS after stating the following: full date, location, number of animals processed, names of each team member.
- Pack the used traps for cleaning and disinfection before they are returned to the transect
- Clean and pack materials used for processing
- Check, arrange and pack samples
- Return to basecamp
FIX BLOOD SMEARS
- Arrange slides face-up on a clean surface and confirm that sample code is clearly visible, if not, trace over to make clear
- Identify a coplin jar with methanol that has no expired
- Open the jar and place smears inside (pairs can be placed back-to-back, MAKE SURE THAT SMEAR IS FACING OUTWARD). Quickly close jar again to prevent the methanol from oxidizing.
- Leave the smears in solution for 5 minutes
- Wearing a pair of gloves, remove each slide and place in an open slide box to dry overnight. Make sure to close the coplin har as quickly as possible to preserve to the methanol
Group all the files from point 21.6 to 21.10 into a unique folder named by the date and range of capture numbers used [yyyy-mm-dd_captures##-##]
SAMPLE SORTING
Organize all samples according to the sample storage protocol.
Unless otherwise indicated by the PI, all other samples should be stored in the freezer until sample intake procedure the next day. NOTE, serum samples must be spun and extracted to a serum storage tube the same night and stored frozen.
Shower and get changed.
Check missing information on the PROCESSING SHEET, and listen to the voice recording to fill in missing information with a differently colored ink pen (usually red).
Upload the information gathered on the PROCESSING FORMS to ODK. Once submitted, check that the laboratory team has all information they need for long-term sample storage
<img src="https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.kxygx9xkdg8j/j87ubx73p2.png" alt="First page of the ODK "MammalCapture" form as it appears on the tablet." loading="lazy" title="First page of the ODK "MammalCapture" form as it appears on the tablet."/>
Write a detailed narrative report of the capture session.
Gather pictures and videos from and sort them into designated folders on the project hard drive
Scan the processing sheets and convert them into PDF files, named by the serial bat capture number.
Retrieve the recording from the voice recorder. Name the file using the following convention "YYYY-MM-DD_SMsession
Clean the traps with with a brush and 10% diluted bleach, rinse them with water and let dry.
Transect traps should be re-baited in evening around 1700 hours. Used traps should be replaced with clean ones.
Re-supply and pack materials for the next capture session



