Handling and Sampling Medium-Large Mammals - ISL Peru
Gideon Erkenswick, Mrinalini Watsa, Jorge Luis Mendoza-Silva, Cristian Tirapelle
Disclaimer
This protocol is actively used by Field Projects International at the Estación Biológico Los Amigos, Madre de Dios, Peru. It is revised annually to reflect improved capture, handling, marking, and sampling methodology. It has been reviewed by the ethics committees of multiple institutions. No author nor affiliated institution takes responsibility or bears any liability for the use of this protocol by others. The protocol is listed as having sensitive content since it involves biosampling from wildlife. Note: these procedures should be carried out only by trained personnel, and are not recommended for use without first obtaining all required permissions.
Abstract
Program Timing
Trap placement and habituation occurs toward the end of the rainy season (March - May). Sample collection occurs annually during the rainforest dry season (June - August). Sample analyses is ongoing between September and April each year.
Team Composition
This protocol is intended to be carried out by a team of 4 individuals, including at least 2 trained personnel. Roles include: (1) designated handler; (2) chemical restraint operator; (3) sampling assistant; (4) data recorder.
Program Overview
Tomahawk live traps are set up at multiple locations encompassing major habitat types surrounding the field station (terra firme, flood plain, swamp, bamboo, successional forest, primary forest, edge forest, etc). Locations are selected based on prior knowledge from the area, camera trap footage, or following standard line transect methodology. Traps may be placed but not activated for a long period of time, until there is evidence that capture of the target animal is likely and predictable.We intend for 10-20 captures each season.
Capture Overview
When a tomahawk trap is baited and activated it is also fitted with a real-time, remote alert system -> medium- to large-size mammals will be chemically restrained with injectable anesthetic agents (10-15 min) -> upon induction, the animal is extracted from the trap for safe processing of morphometric measurements, photographs, nonlethal tissue collection (skin biopsy, fur/nail, blood, mucosal swabs), and placement of microchips or ID tags (20 min) -> an antagonist is administered and the animal is transferred into a covered holding cage, with 3-minute checks for breathing and movement until fully alert (on average ~ 10 - 15 minutes) -> once fully alert, animals are released at the site of capture.
Capture (Additional Details)
Animals are diverse in their behaviors, attraction to bait, and use of forest habitat. Occasionally, for species where capture with a Tomahawk Trap is not possible, such as sloths and anteaters, a capture attempt with the aid of restraining tools may be performed during a chance encounter. In this case, animal processing will be the same as described in this protocol.
Before start
All the material needed for processing the animal should be prepared in advance and the duty of carrying it to the trap site should be distributed among the capture team members.
Steps
ROLES
Team Composition
This protocol is intended to be carried out by a minimum of 4 individuals, including at least 2 trained personnel including: (1) veterinarian (in charge); (2) handler; (3) sampling assistant; (4) data recorder.
Veterinarian in charge (VET)
This is a trained and senior veterinarian that has experience with administering animal anesthesia. This person is responsible for overseeing the monitoring of animal vitals and determining when processing should conclude, even if full sampling has not been achieved. The VET decides whether or not additional doses of anesthesia or emergency drugs are needed, including the time for reversal agent injection.
Handler
This is a trained and senior researcher/veterinarian that is experienced with every step of the capture process. They are responsible for directing sample collection and directly handling the animal along with the VET.
Data Recorder
Will perform data recording on the animal processing sheet MammalForm_hardcopy.pdf
- write down the weight of the animal and any other measurements, sample codes, and important time stamps.
- Recording notes from the HANDLER and VET.
- Notify the team when it is time to check vitals (every 3 minutes from the previous reading),
- make sure that all the vitals are taken at each check.
- Notify the team at 15 minutes from the beginning of the processing of a given animal. | A | B | C | D | | --- | --- | --- | --- | | Discovery (real time, 24hr): | Out of trap (SW): | Recovery (SW): | Release (SW): | | | | | |
Example from the PROCESSING FORM showing important time stamps
Sampling Assistant
Responsible for:
- taking pictures of the animal;
- assisting the HANDLER and VET by passing tools, designated tubes or bags to collect each sample;
- storing samples according to the protocol in use;
- help the DATA RECORDER to double-check that all samples have been recorded on the processing form, and that sample codes on tubes match the PROCESSING FORM.
If more personnel are available, other recommended roles include: (5) PHOTOGRAPHER; (6) COLLAR SPECIALIST; (7) 2ND SAMPLING ASSISTANT.
ACTIVE TRAP MONITORING
At all times , one team member monitors the trap sensor alert system (SAS), while all others remain ready to take action. Shifts should be organized to ensure continuous monitoring of SAS, even during the the night.
When SAS is triggered, the VET and HANDLER depart immediately for the trap-site. They carry with them all essential materials for safe physical and chemical restraint and communication, including (but not limited to):
- cage divider
- drug box
- tarp and handling gloves
- Walkie talkies - used to communicate with the team members that follow, carrying the majority of processing materials (refer to MATERIALS section).
DISCOVERY
The VET and HANDLER approach the trap site first, inspecting the area for signs of any animals around the trap.
If no animals are present in the vicinity of the trap and it is deemed safe, proceed to inspecting the trap.
Visually determine the species and approximate weight (using published reference literature is advised), then cover the entire trap with the tarp. This creates a dark environment that usually reduces stimuli that cause stress and panic.

Communicate discovery details to the rest of the team for processing set up for the particular species
The VOICE RECORDER and the STOPWATCH should be started, and say aloud:
- full date,
- time,
- trap site code/name
- team members participating
Note
From this moment onwards, the time on the STOPWATCH will be the one used when recording the time of any subsequent action on the PROCESSING SHEET.
PREPARATORY PHASE & CHEMICAL RESTRAINT
An estimated weight of the animal should be determined by taking into account guesses from two experienced team members -> prepares the anesthetic according to the pre-selected protocol for that taxa.

Meanwhile, everyone else prepares the PROCESSING TABLE about 30 meters away from the trap.
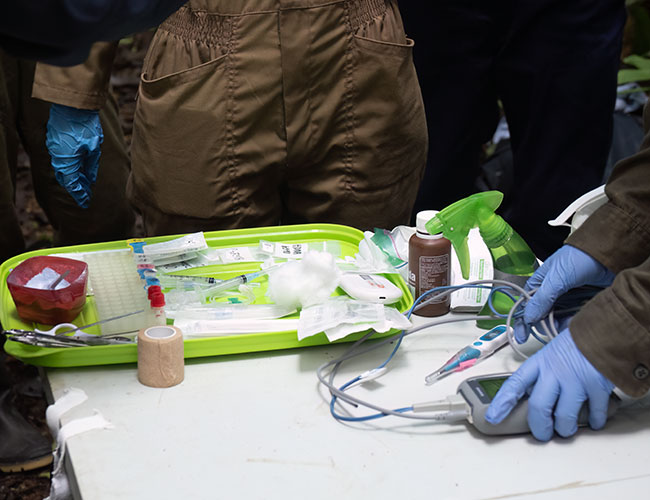
PROCESSING SET-UP:
- clean table surfaces by spraying (in order) with 10% diluted bleach -> water -> 70% ETOH;
- take out enough sampling supplies for the first animal and set on table or in secondary animal tray;
- take out measuring materials;
- ready the tool sterilization kit;
- ensure space for primary animal tray;
- check that camera is ready with lots of free space on card.
CHEMICAL IMMOBILIZATION
Using a trap divider and/or sticks, restrain the animal to one side of the trap while the VET injects the anaesthetic. The VET approaches the side of the trap where the animal’s musculature is closer to the mesh and injects the drug intramuscularly with a SYRINGE or, if neccesary, SYRINGE POLE. The use of a 23G-21G needle has proven to be effective for most medium size mammal species.


UPON INJECTION:
- DATA RECORDER records INJECTION TIME as well as the DOSE for each drug. Failed or partial injections should also be recorded
- start a dedicated STOPWATCH that the VET uses to monitor the duration of chemical immobilization
INDUCTION PERIOD
Cover the trap with a tarp and minimize noise to allow a smooth induction of the anaesthesia. Check on the animal every couple of minutes, or as soon as movement and sounds stop.
When the animal has lowered its head or it is no longer moving any extremities, check for response to external stimuli:
- gently touch a stick to the inner side of the ear pinna,
- check the jaw tone,
- check palpebral reflex.

If no response to stimuli --> open trap, test the reaction of the animal when pulling the paws and lifting the head.
If no response to stimuli --> remove animal and proceed to PROCESSING.
If signs of alertness or arousal to stimuli -> allow more time for the drug to take effect. If 15 - 20 minutes passes since the first dose and the animal is not completely sedated, an additional dose should be calculated and administered.
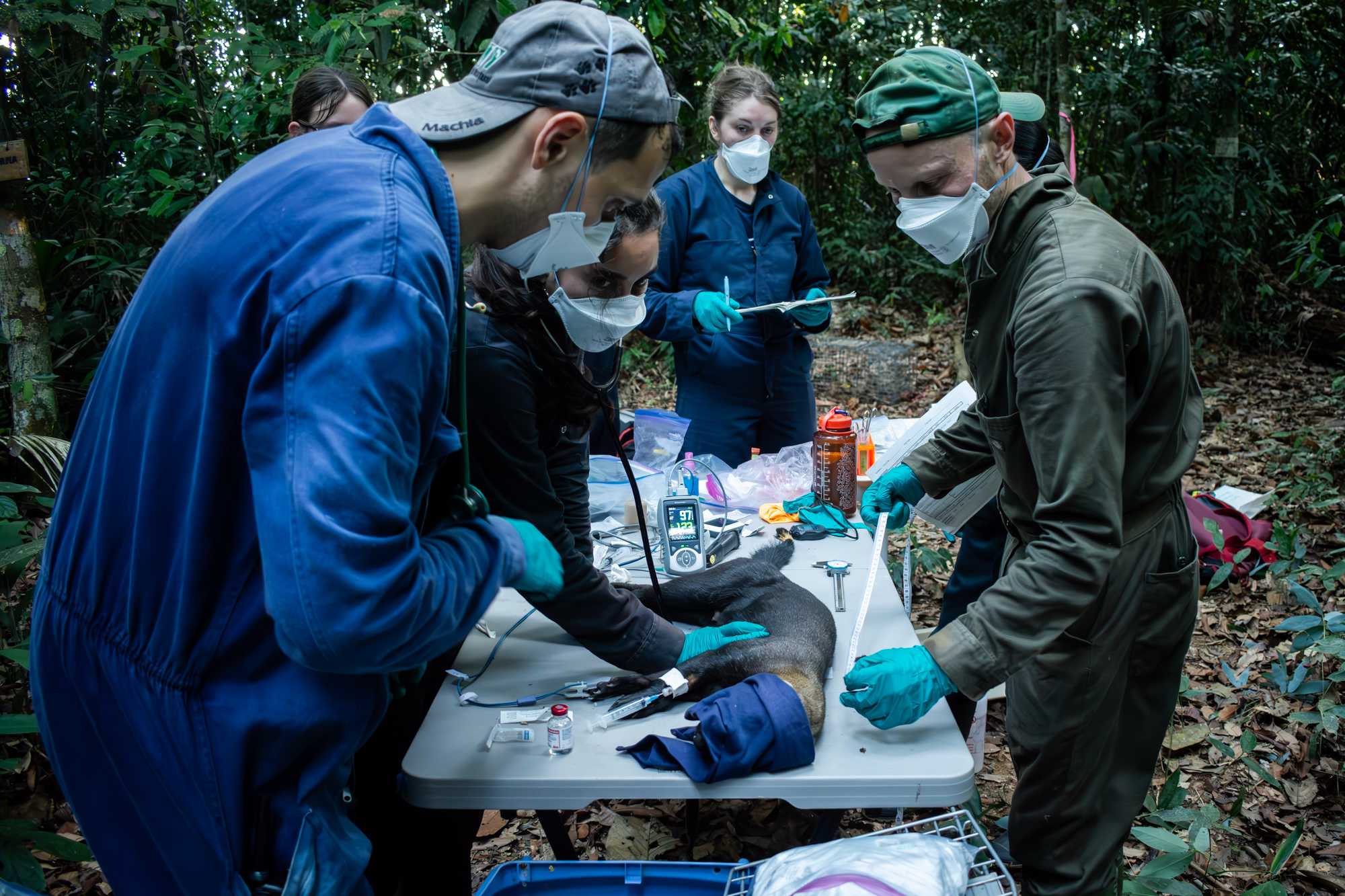
PROCESSING
WEIGHT
Place animal on the tarp to record WEIGHT. Any possible weight estimation error should be taken into account and the VET evaluates whether corrective measures are needed (e.g. additional dosages, reversal agents, etc..).

Meanwhile, sampling assistant mixes activator and hair bleach powder.
Place animal on PROCESSING TABLE with a towel to cover the eyes (this reduces stimuli), and proceed to MEASUREMENTS & SAMPLING.
MEASURMENTS & SAMPLING
The VET and one assistant proceed as follow:
- auscultates the heart sounds and perform the first reading of VITALS.
- places the PUSLOXYMETER on the tongue or the paw pads of the animal
- places THERMOMETER probe intrarectally.
- shaves and prep a site for IV CATHETER (usually the cephalic vein).


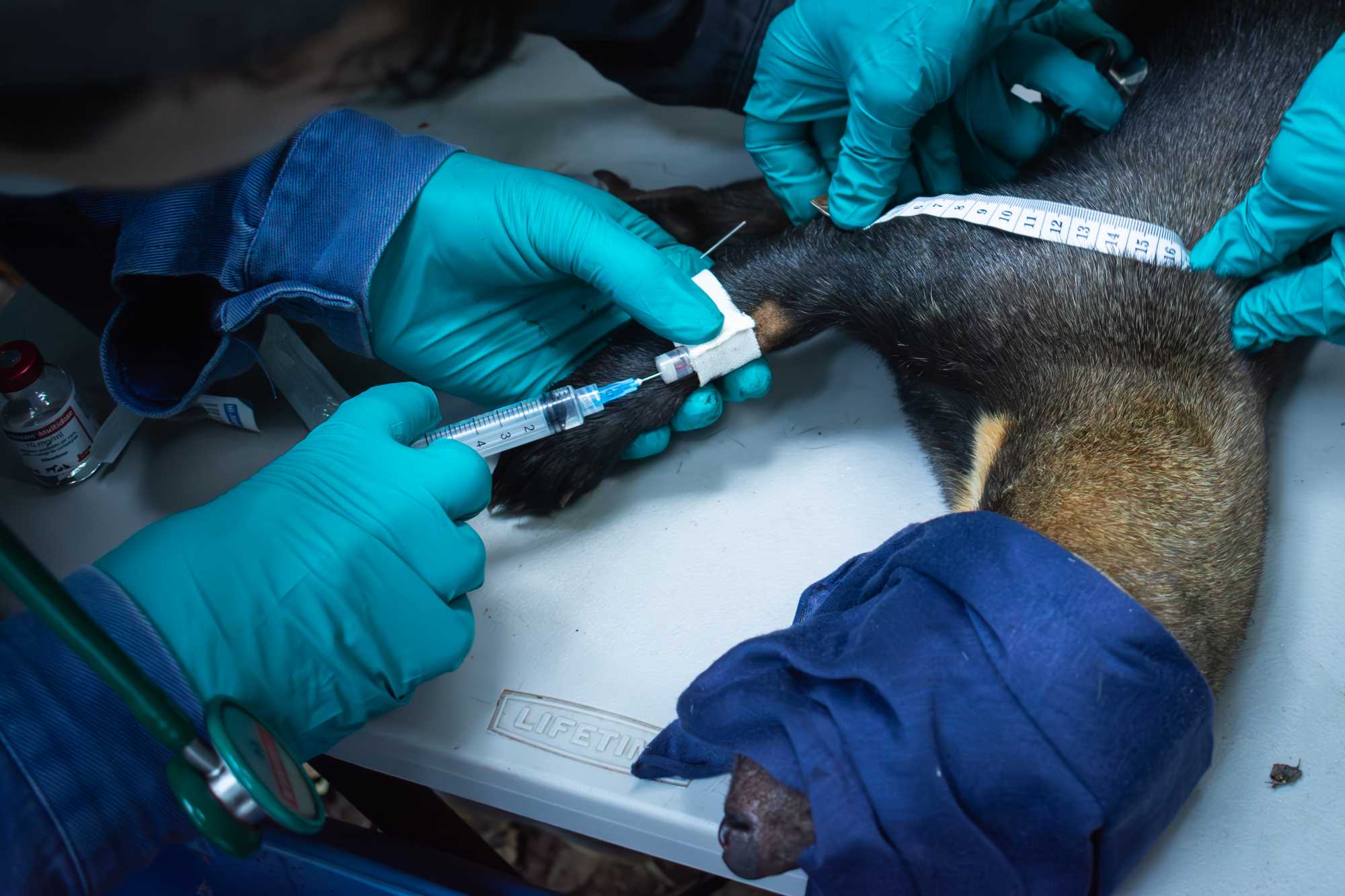
- Place IV CATHETER. The catheter should be left in place for the first 20 minutes of processing. In case of an emergency , the catheter can be used for quick drug delivery (additional anaesthetic drugs VS emergency drugs). If the anaesthesia is uneventful and the processing is about to be completed, the catheter should be removed before the animal will wake up (consider 20-30 minutes from the last effective dose of anaesthetics as a general rule) and the reversal agent is administered.
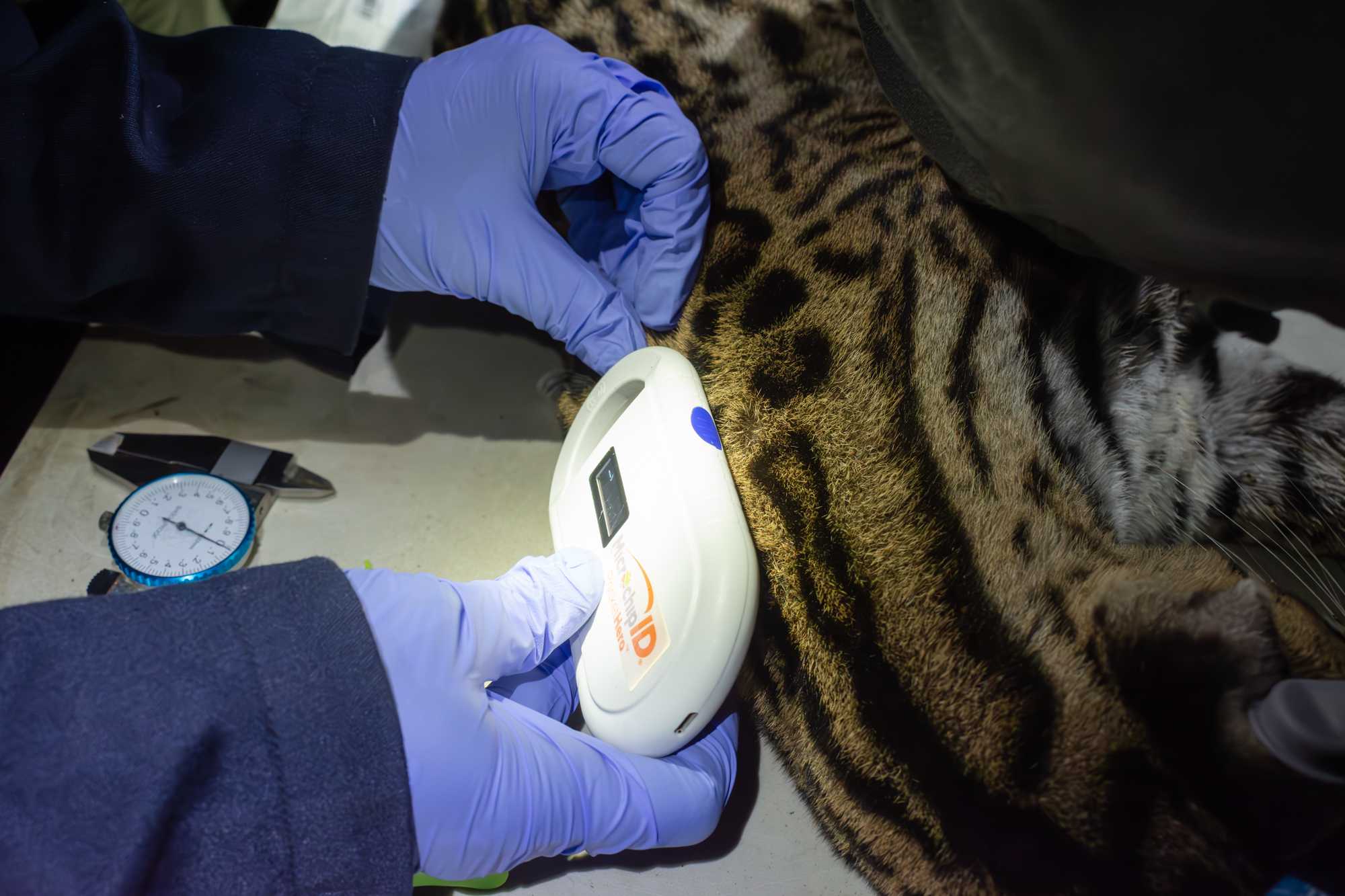
MICROCHIP
- Scan for previous MICROCHIP, if no microchip found, insert one subcutaneously on the dorsal aspect of the neck (use the scanner to check that the chip stays in place after injection).
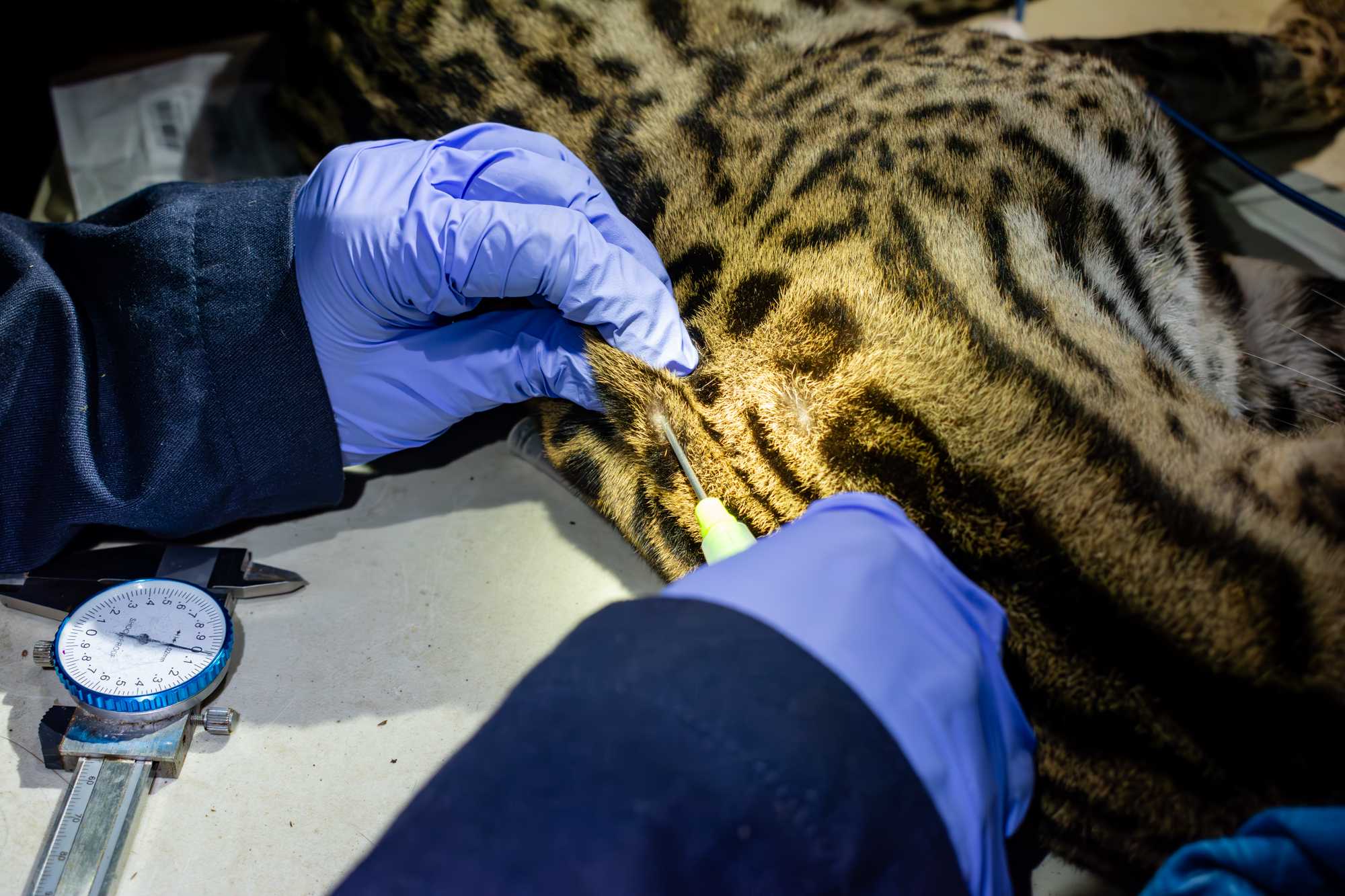
BLOOD
Depending on the species, the blood might be drawn from the jugular, cephalic, femoral, saphenous or ventral coccygeal vein. If blood has not been previously drawn from the IV catether, then:
- shave the selected venipuncture site using a an electric hair clipper,
- disinfect skin with an antiseptic wipe,
- put pressure proximally to the venipuncture site to inflate the vein,
- draw blood using a 3-5 ml syringe with a 23G or 21G needle,
- A drop of blood will be used for blood glucose and ketone readings, and to create 2 blood smears.
0.3mLaliquots of blood are placed in 1.7 mL tubes containing lysis buffer,1mLis placed in tubes containing clot activator factors and another3mLaliquote is placed inside an EDTA tube.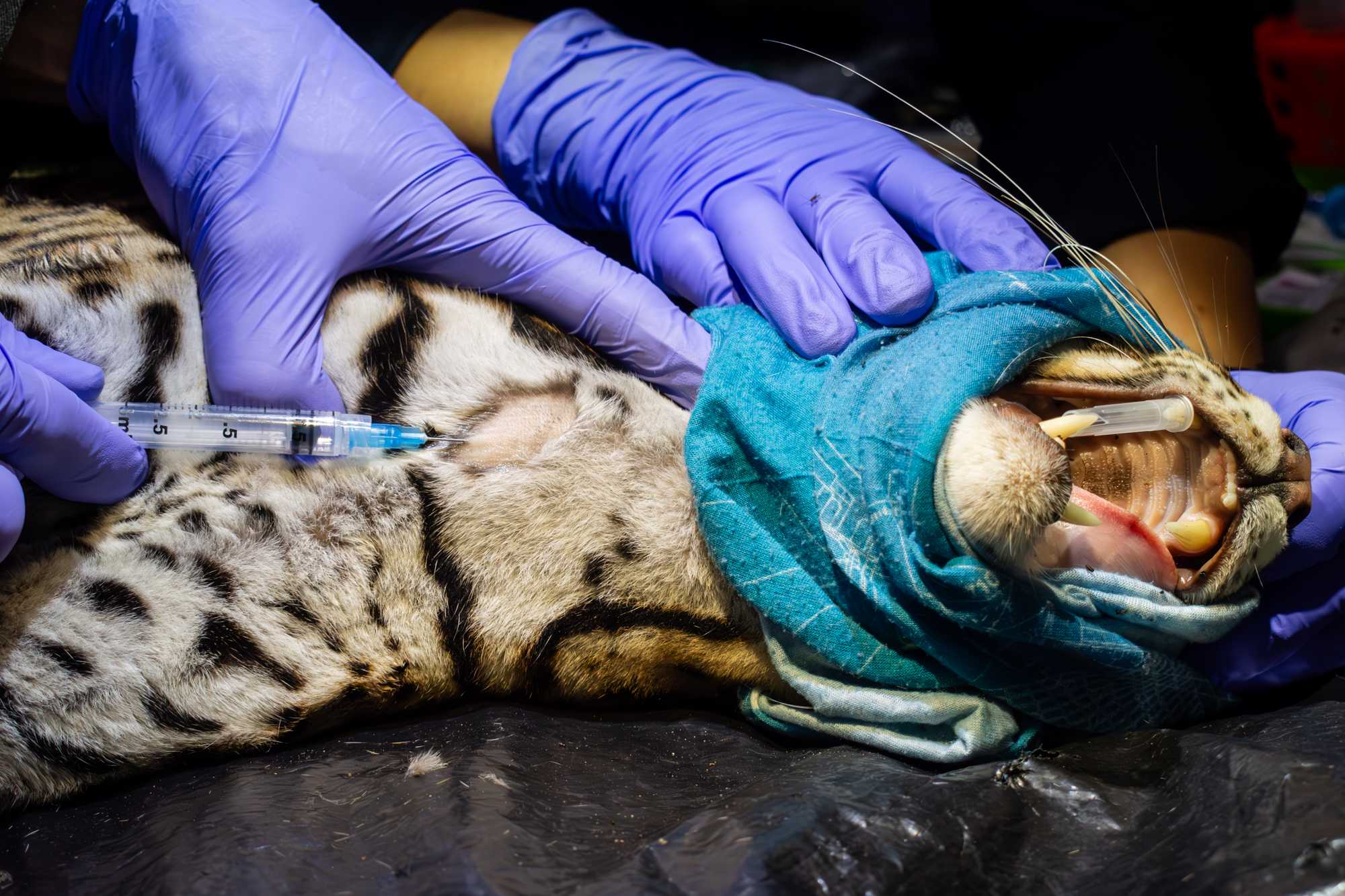
The image shows blood being drawn from the jugular vein of an ocelot. Image courtesy of Jorge Luis Mendoza Silva.
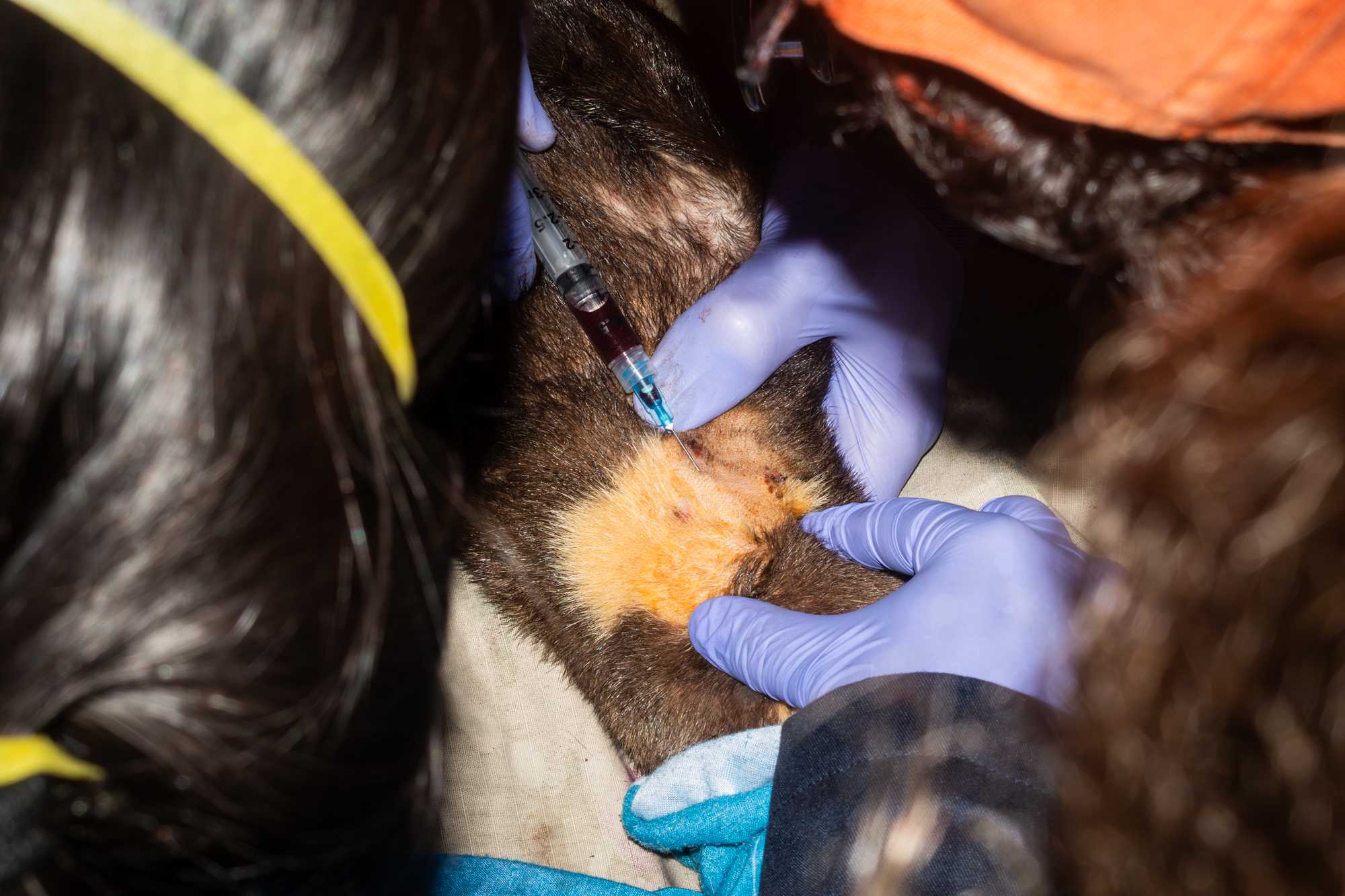
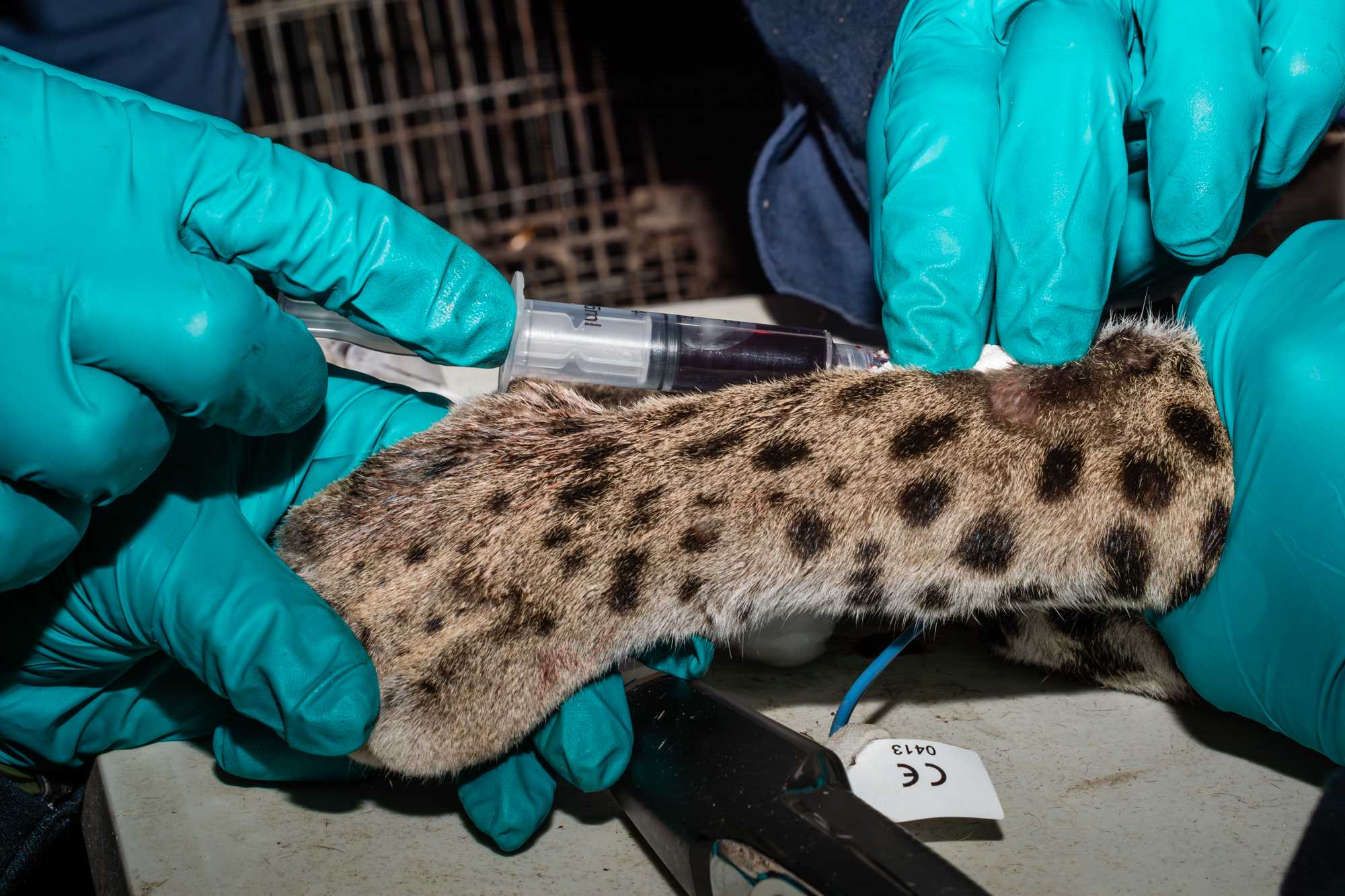
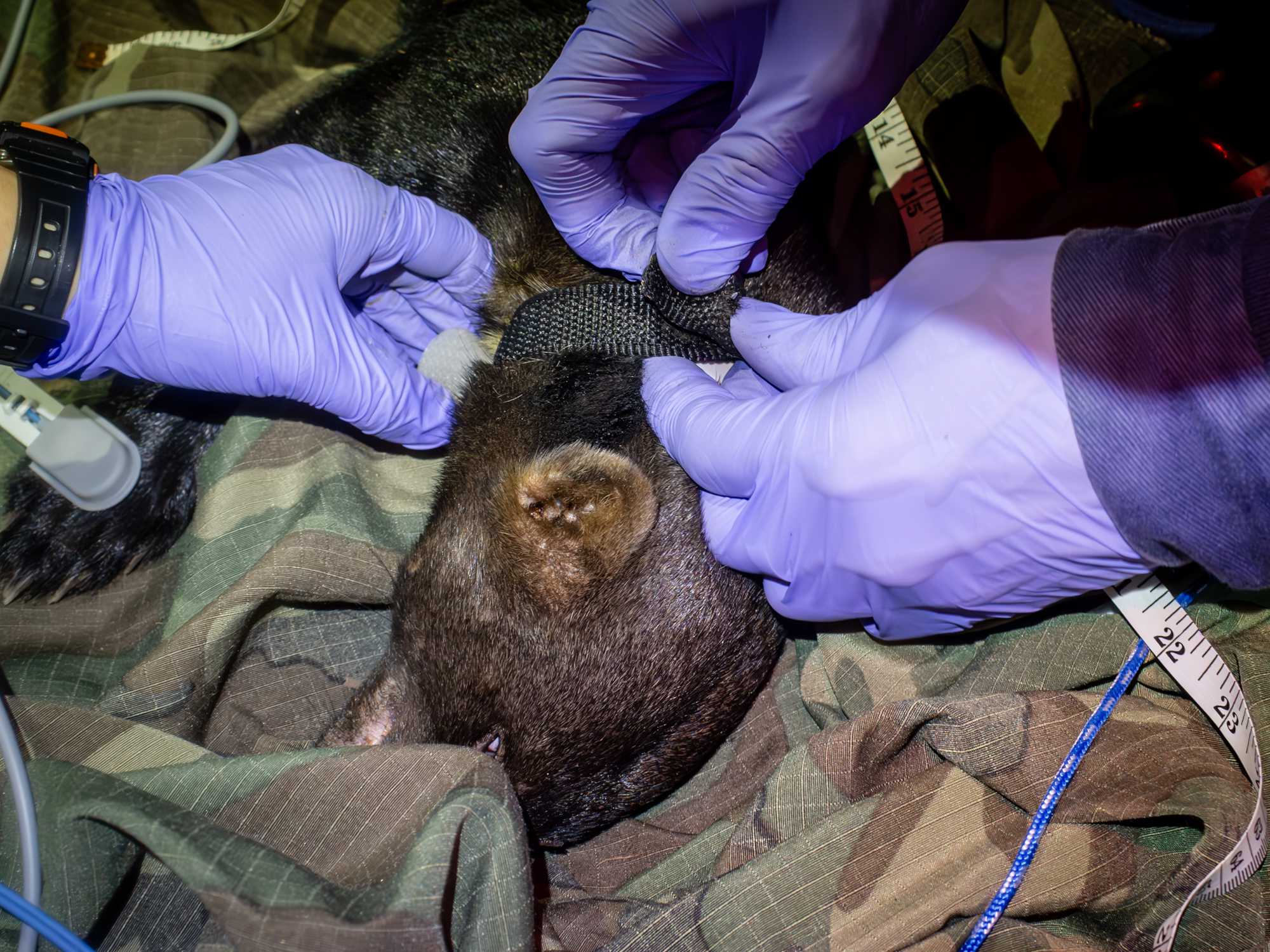
TRACKING DEVICE PACEMENT
Simultaneously to the above operations, team members fit and place a collar tag:
- measure the neck of the animal, marking spots on a string corresponding to where holes for a locking system will be made,
- mark or make corresponding holes in the collar,
- Before locking collar around neck of the animal, collar size should be independently assessed by 2 experienced team members.
PICTURES
The person in charge of pictures will capture:
- PROCESSING SHEET before proceeding to photograph the animal,
- whole animal from one side,
- proceed from head to tail taking all the relevant pictures listed in the PROCESSING SHEET, communicating to DATA RECORDER.
- general pictures of sampling and processing of the animal.
MEASUREMENTS
Person in charge of MEASUREMENTS will:
proceed with BLEACHING the HAIR for marking porpoises following a protocol pre-established for the given species.

- MEASURE all body parts listed in the PROCESSING SHEET from head to tail, communicating each to the DATA RECORDER.
- Searching for scars, injuries (measuring when possible), and ectoparasites.
SAMPLING
Sample collection follows a caudal to cranial order. In an Ideal situation there are two team members working together to collect:
FECES
- Faecal samples are collected from a surface where they have been dropped, directly from the opening of the anus. Look for FECES in the trap, if no feces are found, take two RECTAL SWABS. Use designated, pre-labeled bags or tubes for feces.
VAGINAL SWAB (FEMALE ONLY) x2
- Make sure that the area around the opening of the vulva is clean from faeces,
- access the opening of the vulva without contaminating the swab by touching the fur or the skin in the area, and gently insert the swab directing it towards the birth canal. Be careful not to apply excessive pressure with the swab, and abort if the vaginal does not easily allow swab insertion . Gently rotate the swab back and forth for 5 seconds.
- Remove the swab from the orifice and place it inside the dedicated tube containing lysis buffer
- Hold the swab a few millimetres up from touching the bottom of the tube; this way when you break the tip of the swab inside the tube the piece of swab will be slightly shorter than the length of the tube, and it will fit inside it easily. If needed use a clean scissor to cut the stick.
- Close the tube and place it in the dedicated rake.
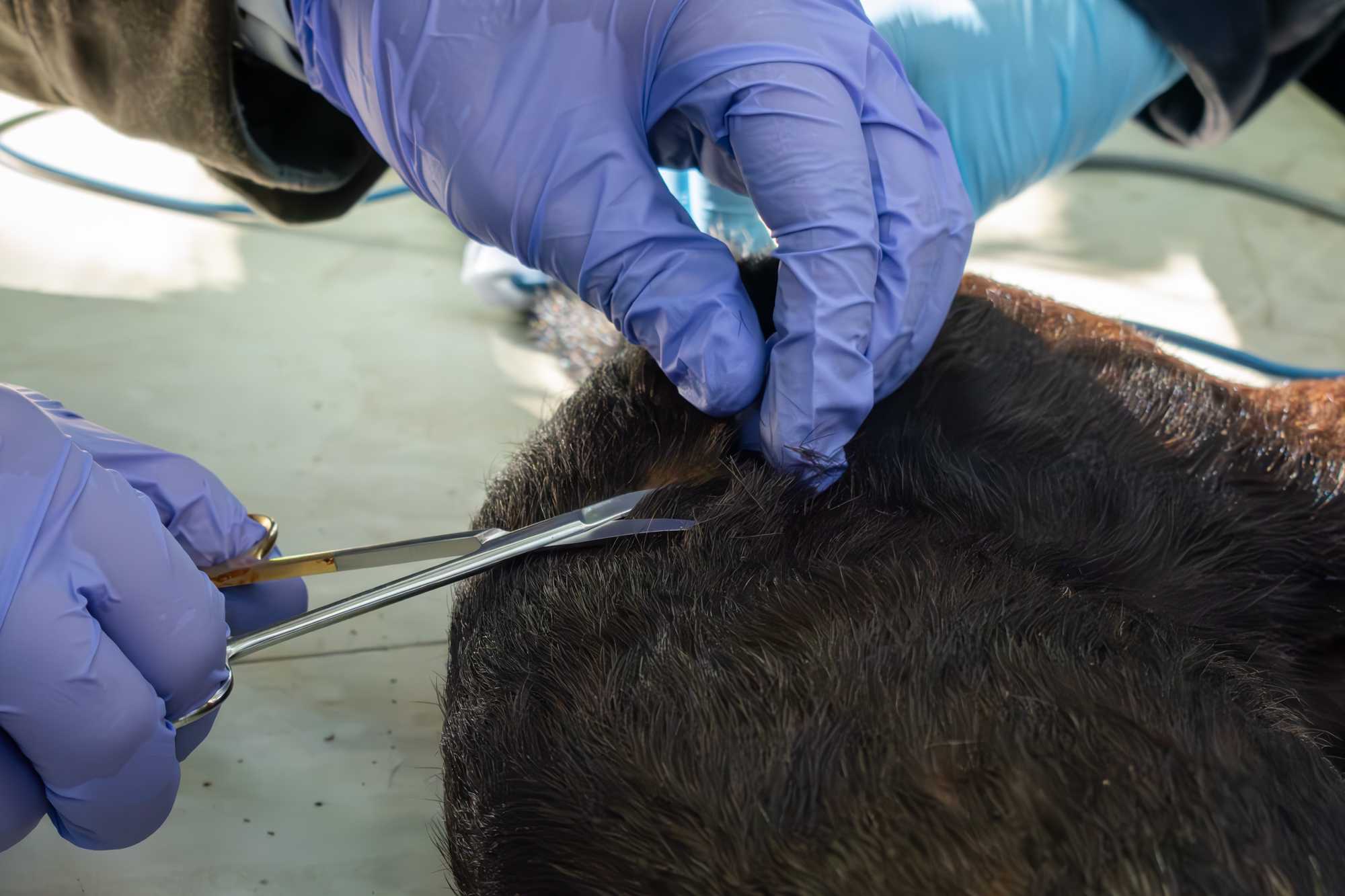
CUT/SHAVED HAIR
- Particularly when working with small animals be careful to avoid accidentally cutting skin.
- To collect hair for mercury studies, cut some hair from the flank or dorsum of the animal. You may also collect hair that is shaved for visualizing the venipuncture site.
- Whenever possible it is good norm to standardize the hair collection site for a species, and keep collecting from the same region in different individuals.
- The body location from which the hair sample is collected should be reported in the processing sheet.
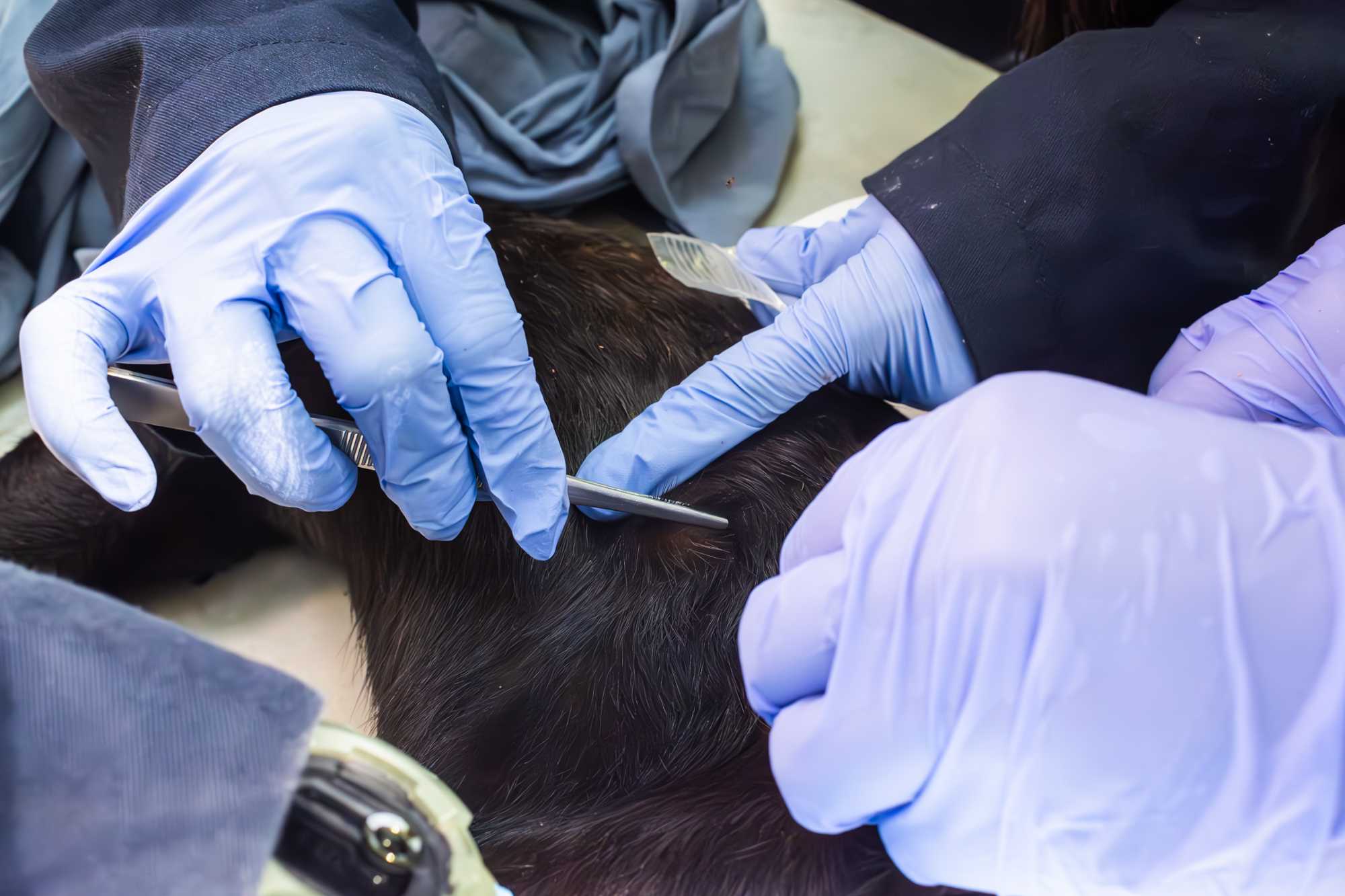
PLUCKED HAIR
- Be careful not to be overzealous and grab too much hair at one time, as (particularly in small mammals) this might injure the skin.
- To collect hair for DNA studies it is important to include the hair follicle in the sample. In order to achieve this, a small amount of hair is grabbed with a pair of tweezers and plucked from the animal's skin. Transfer the hair into a pre-labelled clean zip-lock bag marked as H-DNA followed by a serial number.
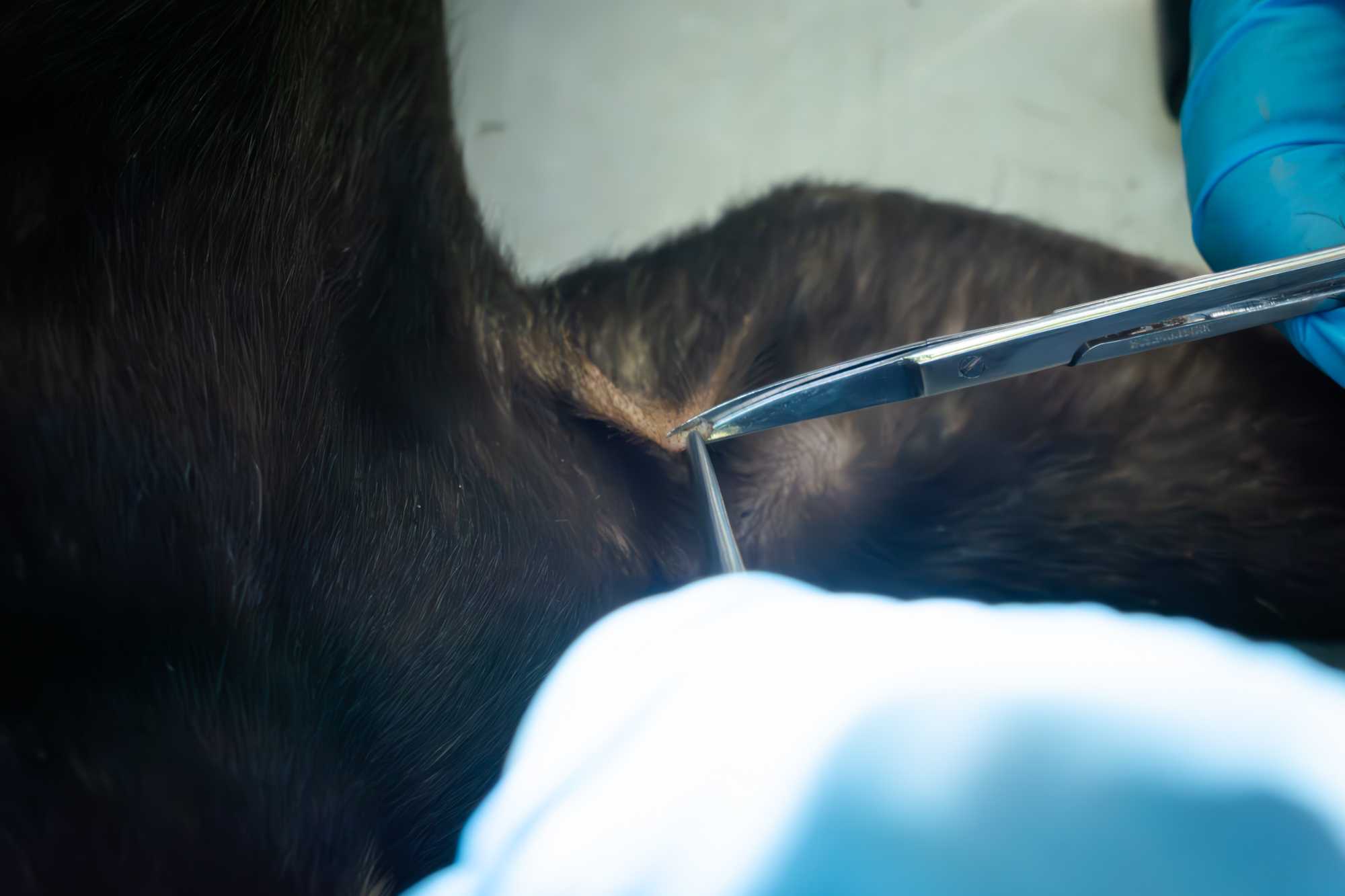
BIOPSY
- Select an area of the skin which is naturally bald, or shave a skin patch on the flank.
- Disinfect the selected skin area with the aid of an antiseptic wipe.
- Grip a small piece of skin (about 3mm) with the tip of a mosquito forceps.
- With a fine scissor cut around the tip of the forceps, this will allow collecting a 0.5cm diameter skin biopsy, to be placed in a dedicated 1.7 mL tube with lysis buffer.
- Place a drop of liquid bandage on the wound left by the biopsy, and let it dry.
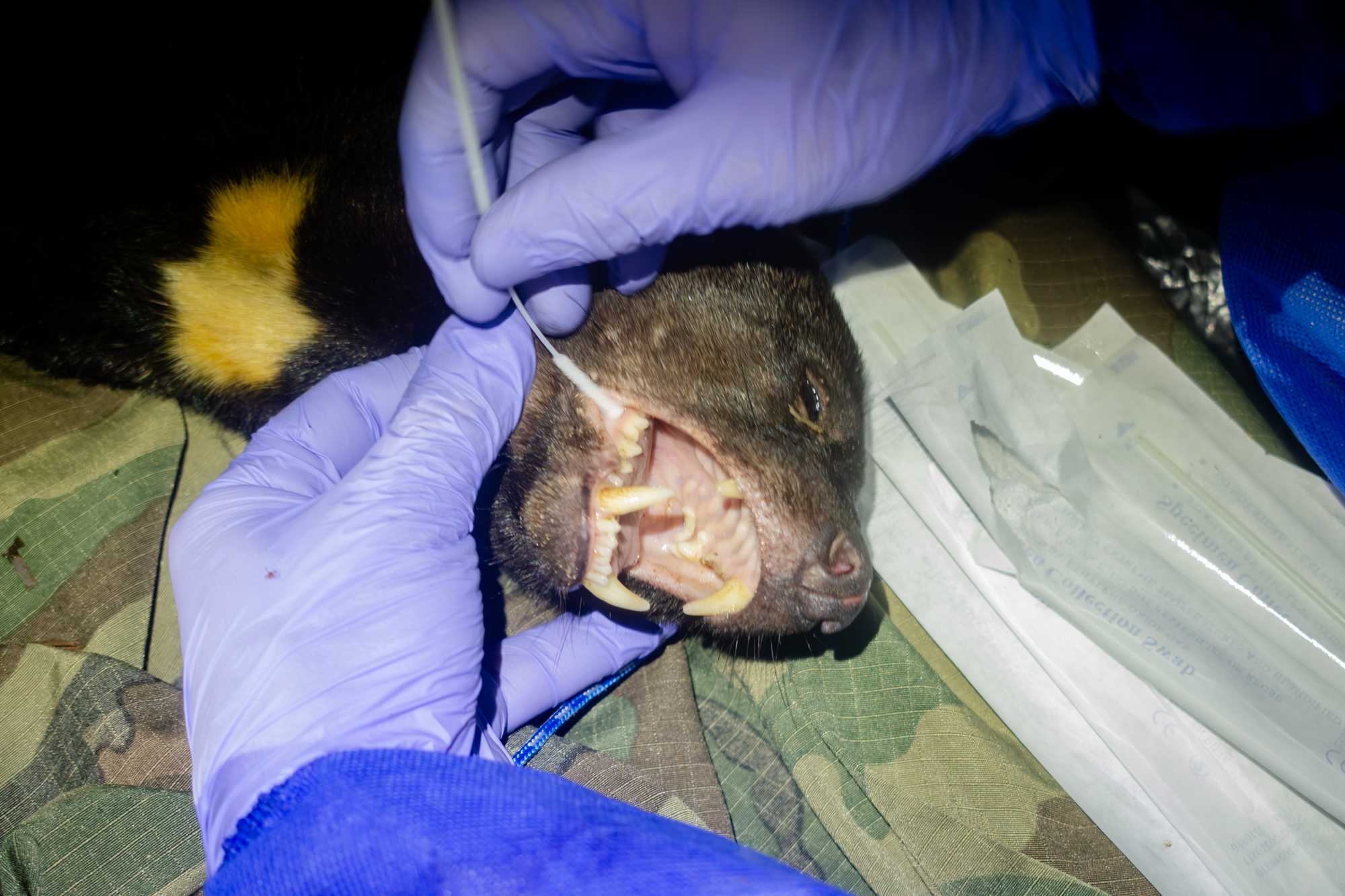
BUCCAL SWAB x2
- Enter the buccal side of the mouth and rub the the swab against the mucous membrane on the internal aspect of the cheek and on the gums. Alternatively, position the swab between the tongue and the gums.
- Rotate the swab back and forth for 5 seconds
- Remove the swab from the orifice and place it inside the dedicated tube containing lysis buffer
- Hold the swab a few millimetres up from touching the bottom of the tube; this way when you break the tip of the swab inside the tube the piece of swab will be slightly shorter than the length of the tube, and it will fit inside it easily. If needed use a clean scissor to cut the stick.
- Close the tube and place it in the dedicated rack.
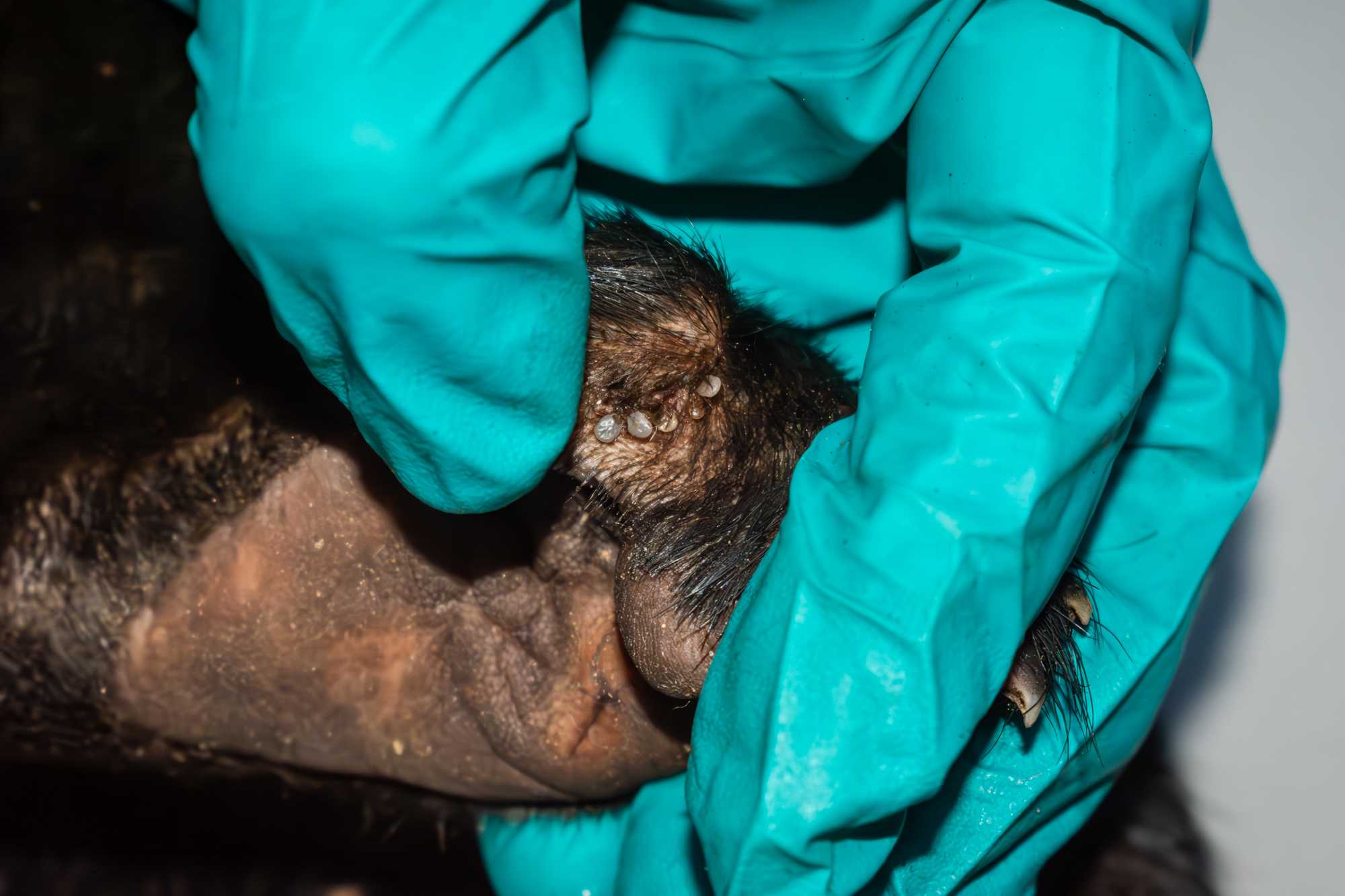
ECTOPARASITES
- The body of the animal, and in particular the interdigital are of the paws, the ventrum, the head and the neck should be inspected for ECTOPARASITES.
- Ectoparasites can be removed from the animal by using a pair of tweezers. In the case you are removing ticks, position the tweezer as close to the animal's skin as possible, aiming to grab the tick from its rostrum and being careful not to inadvertently squeeze the body of the arthropod.
- Once collected, ectoparasites can be stored in tubes containing >= 70% ETOH.
At the end of SAMPLING, the SAMPLING ASSISTANT and DATA RECORDER check that all samples taken have been recorded on the PROCESSING SHEET, and that they match the correct sample codes written on the tubes and bags used.
END PROCESSING & RELEASE
DATA RECORDER reviews PROCESSING SHEET as a checklist for missing data.
No info missing ->
- Remove IV catheter and apply PRESSURE to venipuncture site. VET will consider leaving a PRESSURE BANDAGE in place for 3 minutes to make sure there is no excessive bleeding.
- Removing monitoring equipment from the animal.
- Administer reversal agent intramuscularly (ideally, at least 45 minutes from the last ketamine/anesthetic injection). Record REVERSAL TIME.
- Place animal into the recovery cage, close door, and cover with tarp.
- Reduce noise to ensure smooth recovery.
Only one or two remain close to cage to inspect the animal regularly while it is awaking from the sedation. Presence of standing position, coordinated movements, interest in the bait* and managing to stimulate defensive behaviour when poking the animal, are all signs that can be used to evaluate the level of readiness for the release.
Meantime, the rest of the team may reorder samples and processing materials, generally preparing for departure to basecamp.
Once animal recovers, record WAKE UP TIME, and a person may take position to safely capture pictures and videos of the release.
The door of recovery cage should face an open area, clear from people and away from water, cliffs or any other dangerous areas.
Open the door with protective gloves, or from a distance by pulling a rope attached to it.
Record RELEASE TIME on the STOPWATCH as well as any relevant comment on animal behaviour during and after release.
END SESSION
FIELD WRAP UP
After RELEASE:
- End the VOICE RECORDER after stating the following: full date, location, number of animals processed, names of each team member.
- Pack the used traps for cleaning and disinfection before they are returned to the transect (e.g., spraying with atoxic disinfectants).
- Clean and pack materials used for processing.
- Check, arrange and pack samples.
- VET must score the quality of the induction, maintenance, recovery, muscle relaxation achieved with the anaesthetic protocol, and give an overall assessment of chemical immobilization that is indicated on the PROCESSING SHEET.
- Clean out trap and return it to the same position as it was for baiting. Open, insert bait (if available, and ensure it is deactivated. Turn on any camera traps that record visits to the trap or surrounding area.
- Return to base camp.
FIX BLOOD SMEARS
- Arrange slides face-up on a clean surface and confirm that sample code is clearly visible, if not, trace over to make clear.
- Identify a coplin jar with methanol that has not expired.
- Open the jar and place smears inside (pairs can be placed back-to-back, MAKE SURE THAT SMEAR IS FACING OUTWARD). Quickly close jar again to prevent the methanol from oxidizing.
- Leave the smears in solution for 5 minutes.
- Wearing a pair of gloves, remove each slide and place in an open slide box to dry. Make sure to close the coplin jar as quickly as possible to preserve to the methanol.
Group all the files from point 21.6 to 21.9 into a unique folder named by the date and range of capture numbers used [yyyy-mm-dd_captures##-##].
SAMPLE SORTING
Organize all samples according to the sample storage protocol.
Unless otherwise indicated by the PI, all other samples should be stored in the freezer until sample intake procedure the next day. NOTE, serum samples must be spun and extracted to a serum storage tube the same night and stored frozen. EDTA tubes shall be moved to the -80 degree freezer soon after their intake.
Shower and get changed.
Check missing information on the PROCESSING SHEET, and listen to the voice recording to fill in missing information with a differently colored ink pen (usually red).
Upload the information gathered on the PROCESSING FORMS to ODK.
<img src="https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.81wgby8y1vpk/image.png" alt="First page of the ODK "MammalCapture" form as it appears on the tablet." loading="lazy" title="First page of the ODK "MammalCapture" form as it appears on the tablet."/>
Write a detailed narrative report of the capture session.
Gather pictures and videos from and sort them into designated folders on the project hard drive.
Scan the processing sheets and convert them into PDF files, named by the serial mammal capture number.
Retrieve the recording from the voice recorder. Name the file using the following convention "YYYY-MM-DD_LMsession
Resupply and pack materials for the next capture session.

