Handling and Sampling Bats - ISL Peru
Gideon Erkenswick, Mrinalini Watsa, Jorge Luis Mendoza-Silva, Cristian Tirapelle, Priscila Peralta-Aguilar, Naija Cuzmar, Pamela Sánchez-Vendizú
Disclaimer
This protocol is actively used by Field Projects International at the Estación Biológico Los Amigos, Madre de Dios, Peru. It is revised annually to reflect improved capture, handling, marking, and sampling methodology. It has been reviewed by the ethics committees of multiple institutions. No author nor affiliated institution takes responsibility or bears any liability for the use of this protocol by others. The protocol is listed as having sensitive content since it involves biosampling from wildlife. Note: these procedures should be carried out only by trained personnel, and are not recommended for use without first obtaining all required permissions.
Abstract
Program Timing :
Sample collection occurs annually during the rainforest dry season (June - August). Sample analyses occur between September and April.
Program Overview :
Bat mist nets are set up at multiple locations encompassing major habitat types surrounding the field station (terra firme, flood plain, swamp, bamboo, successional forest, primary forest, edge forest, etc). Mist netting occurs over 3-4 consecutive days at each location throughout the sample collection period. Dependent on resource availability and personnel, we strive for an annual sample size of 200 - 300 bats.
Team Composition
This protocol is intended to be carried out by a team of 3 individuals, including at least 2 trained personnel. Roles include (1) designated bat handler (2) sampling assistant (3) data recorder. Modification of team composition should be made according to the anticipated number of captures.
Capture Overview :
Mist nets are installed a day prior or several hours ahead of an intended capture event -> nets are opened between 17:00 and 22:00 (generally), and captures occur spontaneously throughout (~ 5 - 20 animals/event) -> animals are extracted from net (1-2 minutes) -> transferred into a light and breathable cloth bag and kept in order-of-capture in a nearby tent until processing (0 - 30 minutes) -> one-by-one animals are processed for morphometric measurement, photographs, marking (optional) and nonlethal tissue collection (~10 minutes) -> animals are immediately released at the site of capture once processing is complete, but away from mist nets.
Before start
The PI is in charge of coordinating the bat capture activities and selecting the sites where mist nets are placed. Sites are changed every 3 - 4 days to avoid high recapture rates, or as determined by the PI.
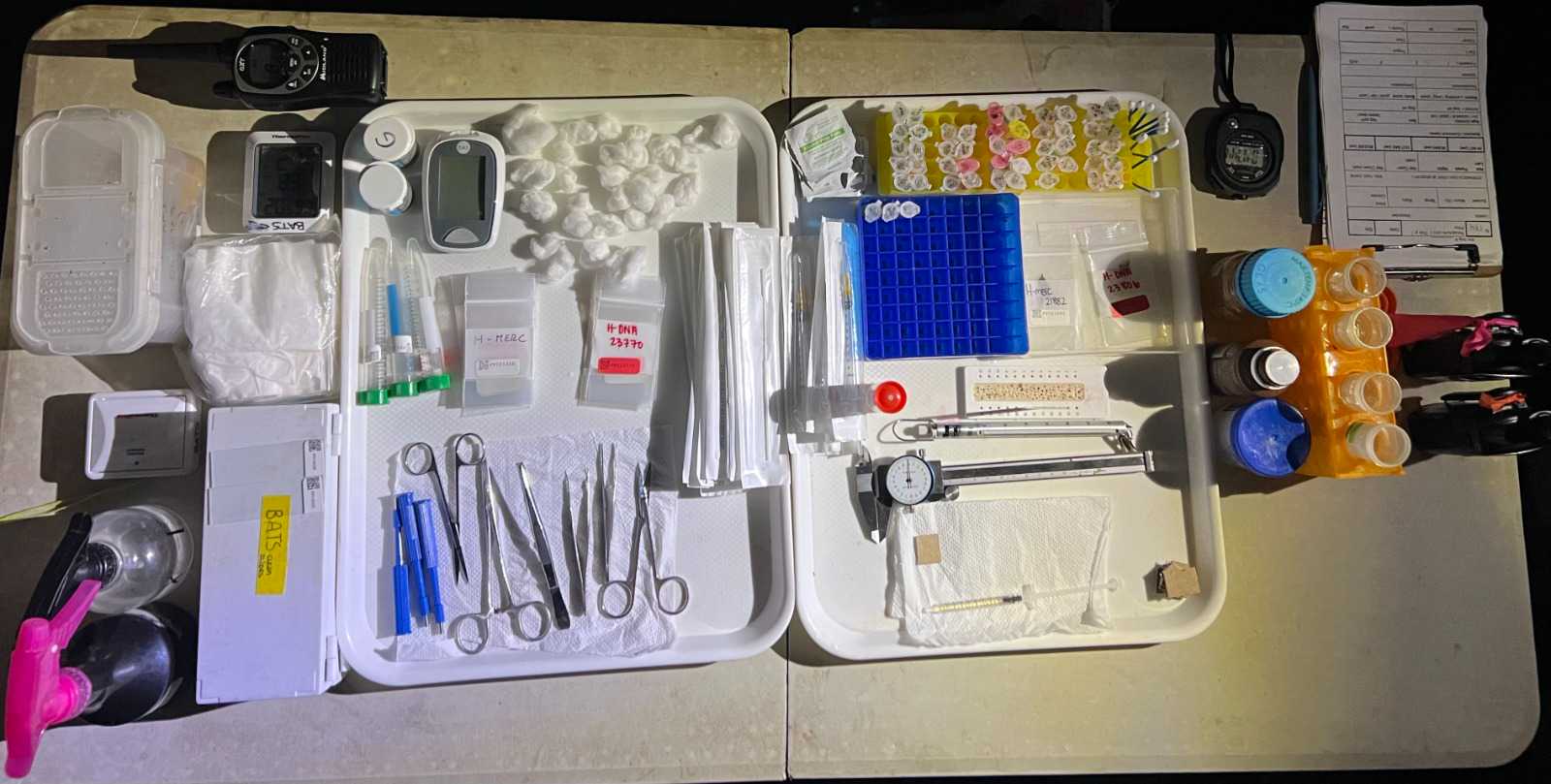
Bat team personnel will pack and double-check that all capture materials needed are cleaned and organized before departing to the capture site. Refer to the MATERIALS section.
Steps
ROLES
Team Composition
This protocol is intended to be carried out by a team of 3 individuals, including at least 2 trained personnel. Modification of team composition should be made according to anticipated number of captures. Roles include, (1) designated animal handler (2) sampling assistant (3) data recorder.
Designated Handler
This is a trained and senior researcher/veterinarian that is experienced with every step of the bat capture process. They are responsible for animal mist-net removal, as well as direct handling during the processing phase and release.
Sampling Assistant
Will assist the designated handler with:
- Passing tools, bags and tubes needed for processing
- Reading aloud the serial codes that identify each sample bag and tube.
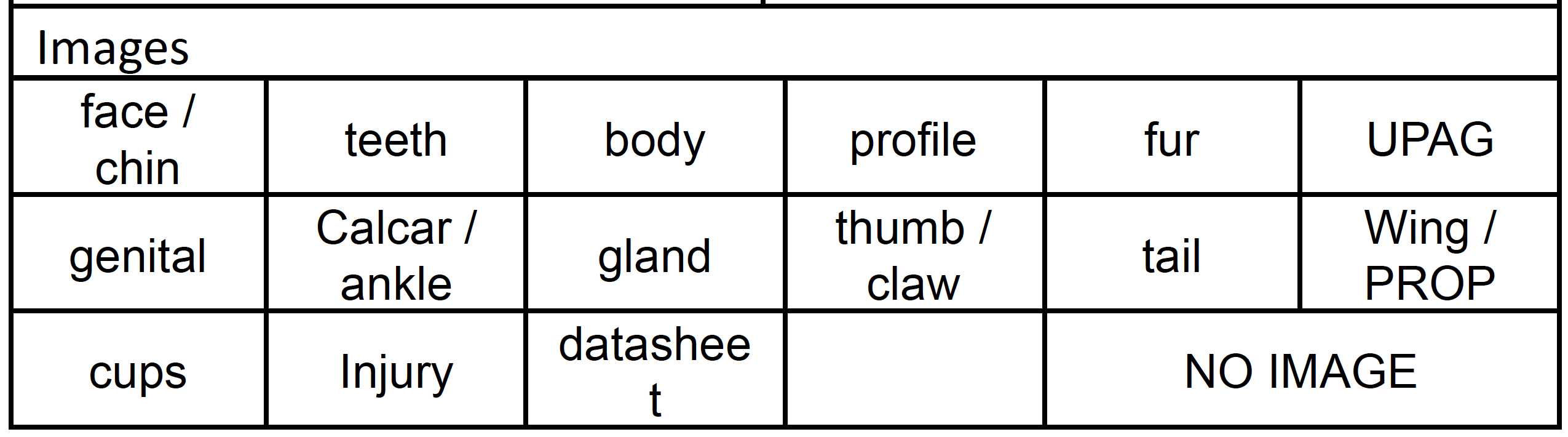
- Taking all images listed in the processing sheet.
Data Recorder:
Will perform data recording on the animal processing sheetBatsForm_hardcopy.docx.pdf :
- Write down the weight of the animal and any other measurements, sample codes, and important time stamps.
- Recording notes from the handler
- Storing samples in their dedicated sample boxes.
PREPARATORY PHASE
Bat team personnel pack and double-check all bat capture and processing materials needed before departing to the capture site. Refer to the MATERIALS section.
One day prior, the team will install 4-12 nylon mist nets (6 - 12 x 2.7 m, 4 - 5 shelves, mesh size 15 - 20 mm) in the chosen areas. The number of mist nets used should reflect with the size of the bat team and frequency of bat capture at that particular site. Nets must be closed if:
- inadequate personnel are available to adhere to expected processing times
- weather conditions are not poor
- or to reduce inspection time when temperatures are low
Generally, nets remain activate from 17:00 to 23:00.
A screened-in tent with a table or processing cot is setup within 25 - 50 meters of the mist nets, which will be used for processing animals.
CAPTURE
The nets are opened from sunset, typically around 17:30 hours, until 22:00 hours and checked according to the following schedule:
- First 2 hours - every 20 minutes
- After 2 hours - every 30 minutes, if the rate of capture is low
- Cold temperature - every 15 minutes if air temperature is below 20 degrees Celsius
Start the STOPWATCH and VOICE RECORDER, which should be running during the entire session. Say aloud: (1) the full date and time; (2) the weather and temperature; (3) the capture site; (4) the team members participating; (5) total number of nets; (6) the times on the stopwatch and time elapsed on the recording.
Important times recorded on the processing sheet should refer to the time indicated on the stopwatch.
NET INSPECTION:
The person(s) inspecting the nets carry a walkie-talkie to communicate with the rest of the team waiting inside the tent. As soon as a captured bat is discovered the team is informed so that DISCOVERY TIME, NET NUMBER and PANEL NUMBER may be recorded.
BAT REMOVAL:
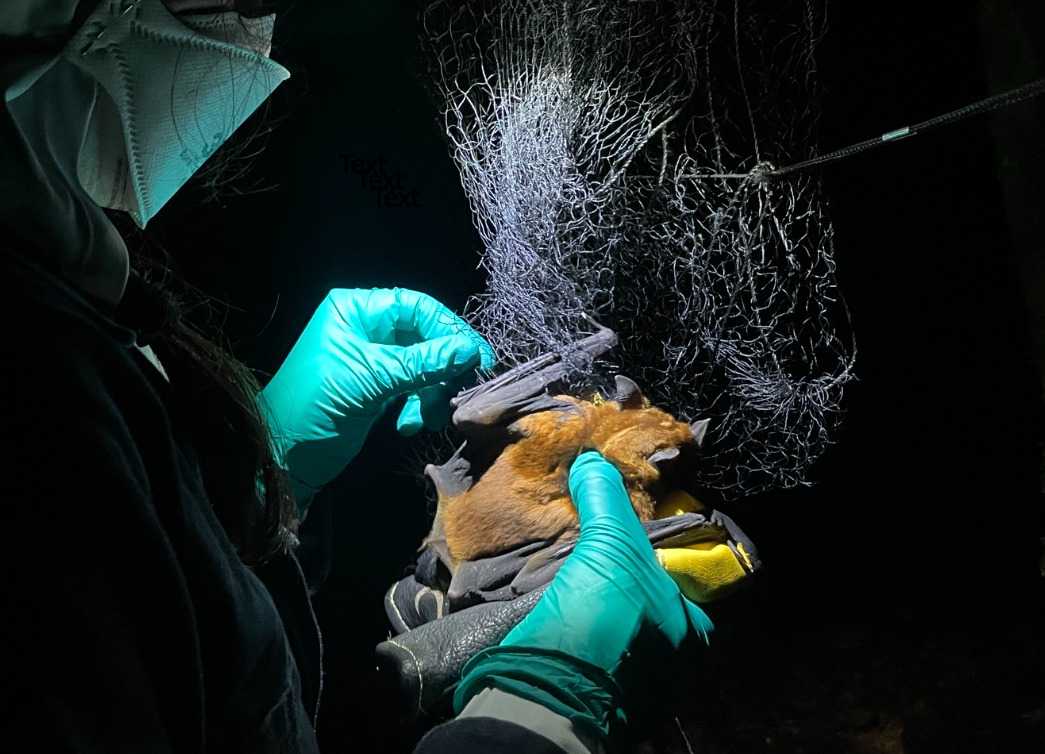
Only designated and experienced personnel may remove the bats from the net:
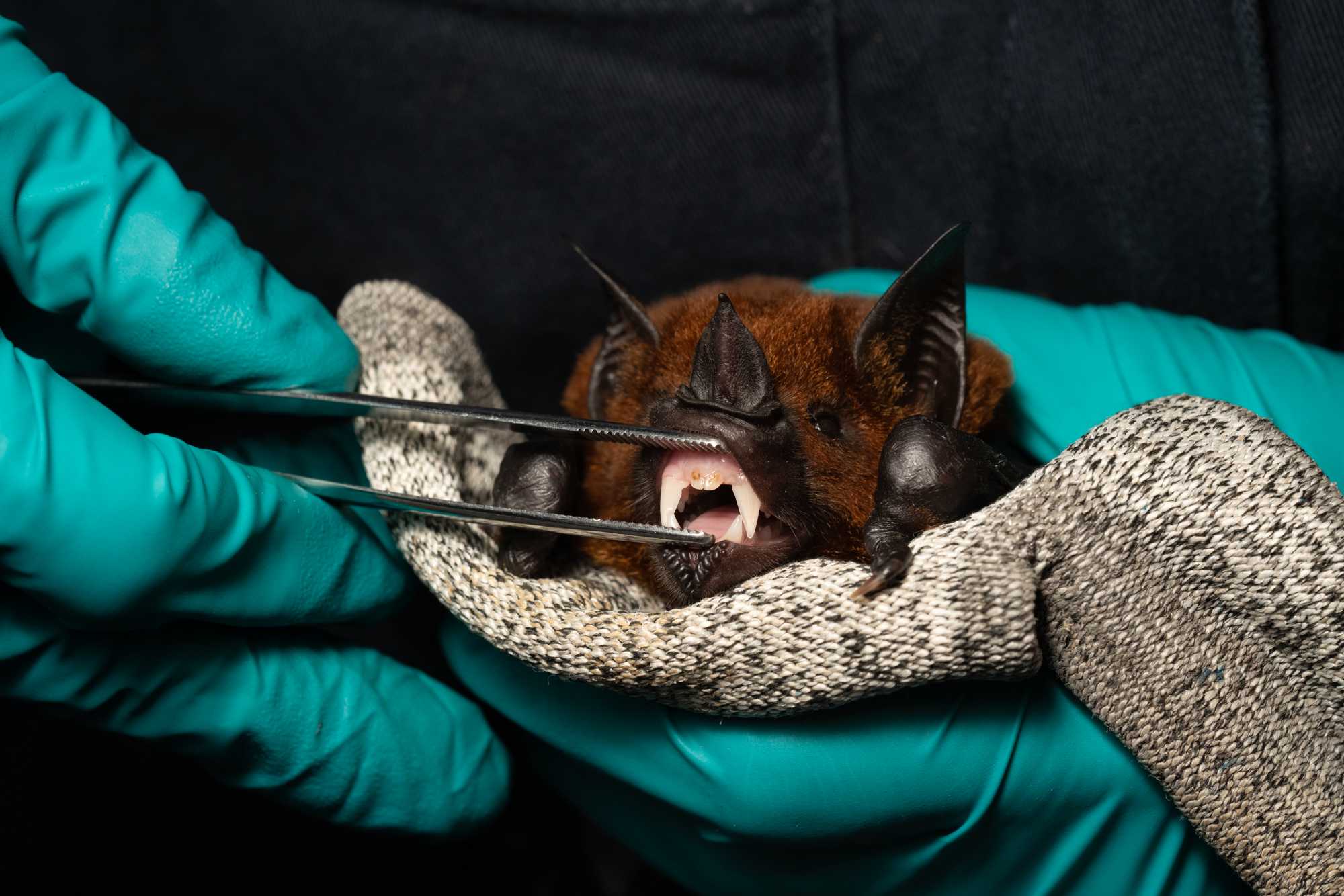
- Place a clean thick leather glove in between the ventrum of the bat and the palm of the non-dominant hand. The bat is restrained between the palm and the thumb. The thumb is positioned on the bat's dorsum and pushes the bat gently against the glove. CAREFUL NOT TO COVER THE HEAD OF THE BAT, WHICH COULD CAUSE SUFFOCATION. See image below.
- Using the dominant hand, gently pull the mesh of the net away from the feet and legs, untangle the wings, and lastly the head.
- Once removed, the bat is placed inside a clean cloth-bag and brought to the processing tent. The BAG NUMBER should be written on a piece of masking tape attached to the bag, and recorded on the ANIMAL SHEET.
Record the time that the animal is placed inside a cloth-bag (IN BAG TIME)
An animal is processed straight away or left on a line and processed in order of net extraction, however, always give first priority to bats that appear depressed. The waiting line must be in a DRY place that is not exposed to wind.
Bats waiting in bags must be checked for signs of movement/life every 10 minutes. If an animal appears unwell the team leader must be notified immediately.
PROCESSING
Record the weight of the cloth holding bag + bat inside
Designated personnel will remove the bat from the bag and handle the animal for the entirety of processing.
Carefully open the bag where the bat is kept, identify the head of the animal, and position it so that the head is facing away from you.
SAMPLING
CUT/SHAVED HAIR
Before cutting, identify the length of the hair on the dorsum by pulling it back with the scissors closed. This action will help to show the maximum length that can be cut to avoid cutting skin. Then, with a fine scissor held parallel to the dorsum cut HAIR and place it in a H-merc bag.
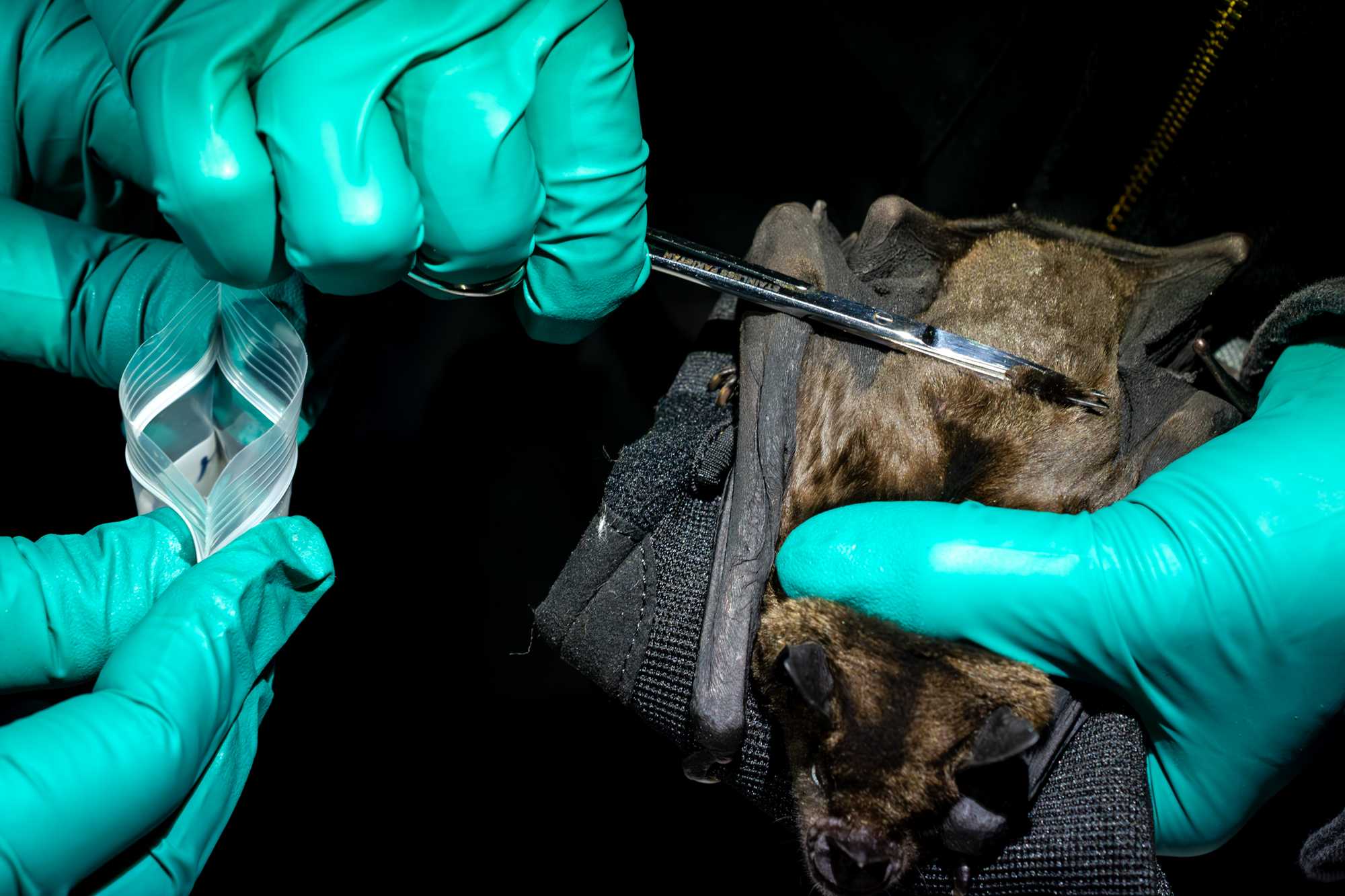
WING BIOPSY
Unfold one of the wings (usually the right one), and place it against a light source (we recommend a light pad such as this one). Mark a small dot at a non-vascularized area of the dactylopatagium with a black marker (sharpie).
Move the wing against a sterile piece of cardboard that provides a firm surface for extracting a clean biopsy.
Disinfect the area with an antiseptic wipe.
Collect a 3mm biopsy-punch sample using a clean biopsy punch.
Store the biopsy in a buffer that will not lyse the sample. Avoid Zymoshield, longmires, etc.
Take the hole punch in the same location for all bats you work with to ensure easier recognition of recaptured animals at the same site in the next few days.
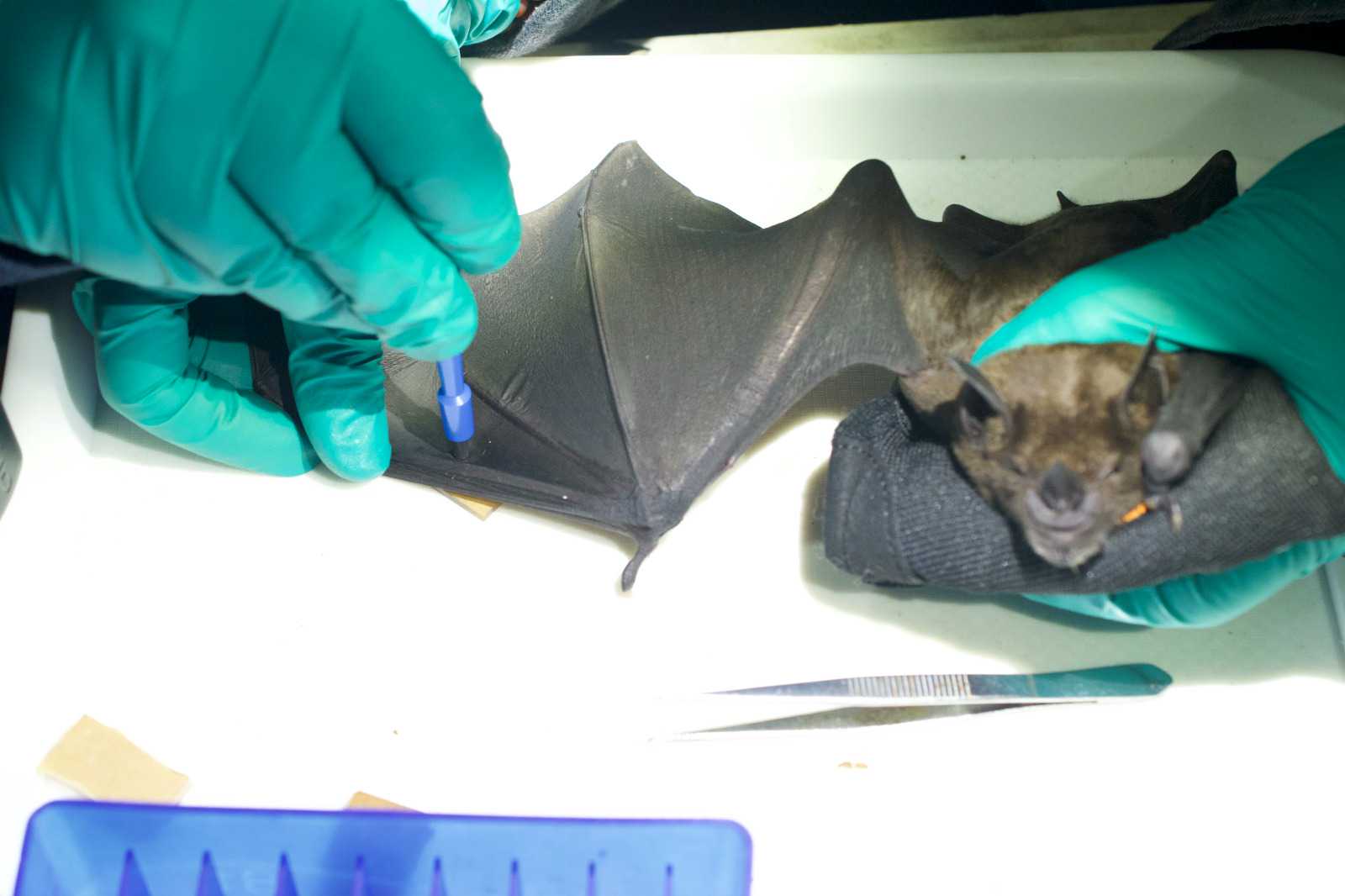
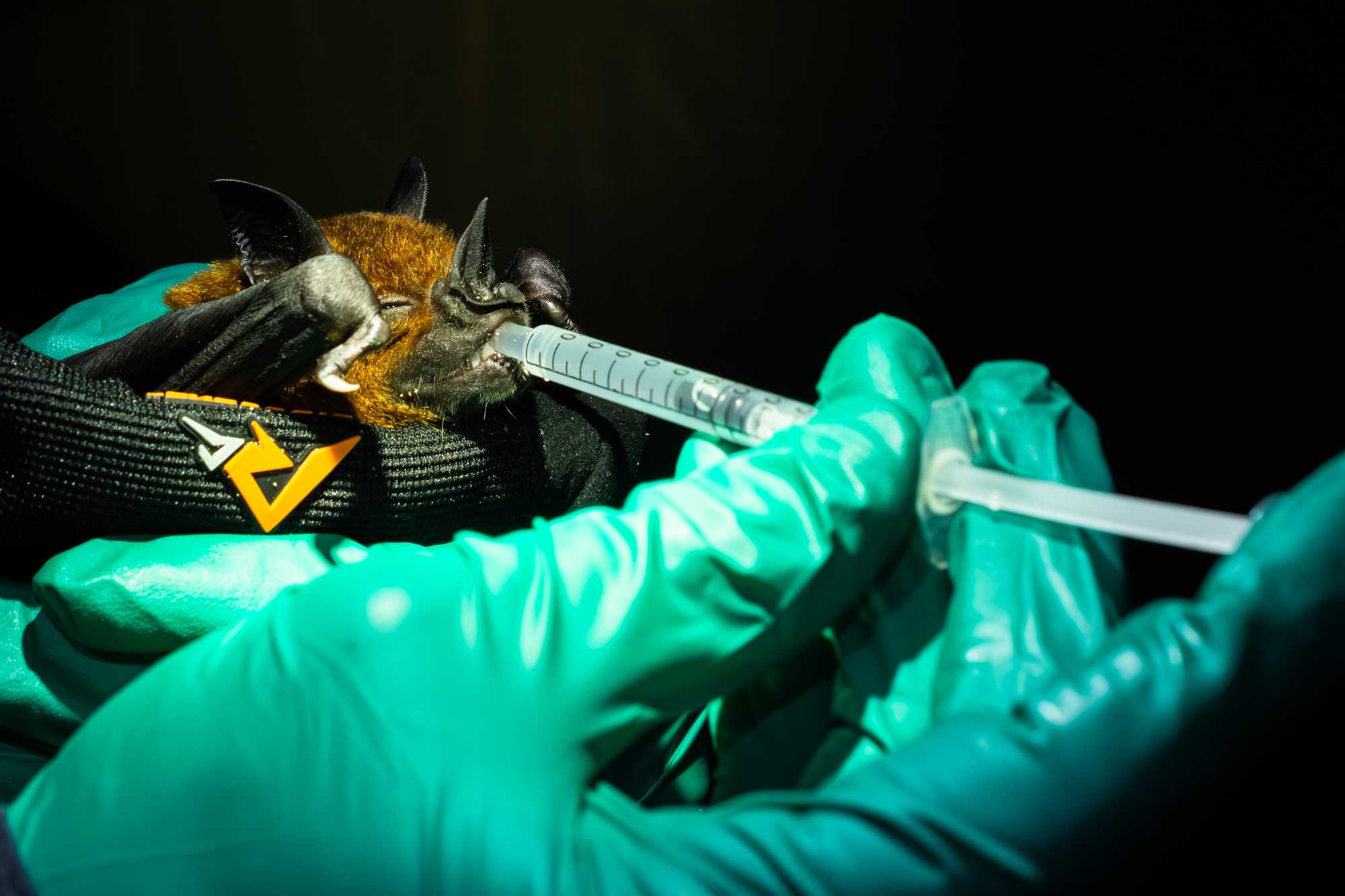
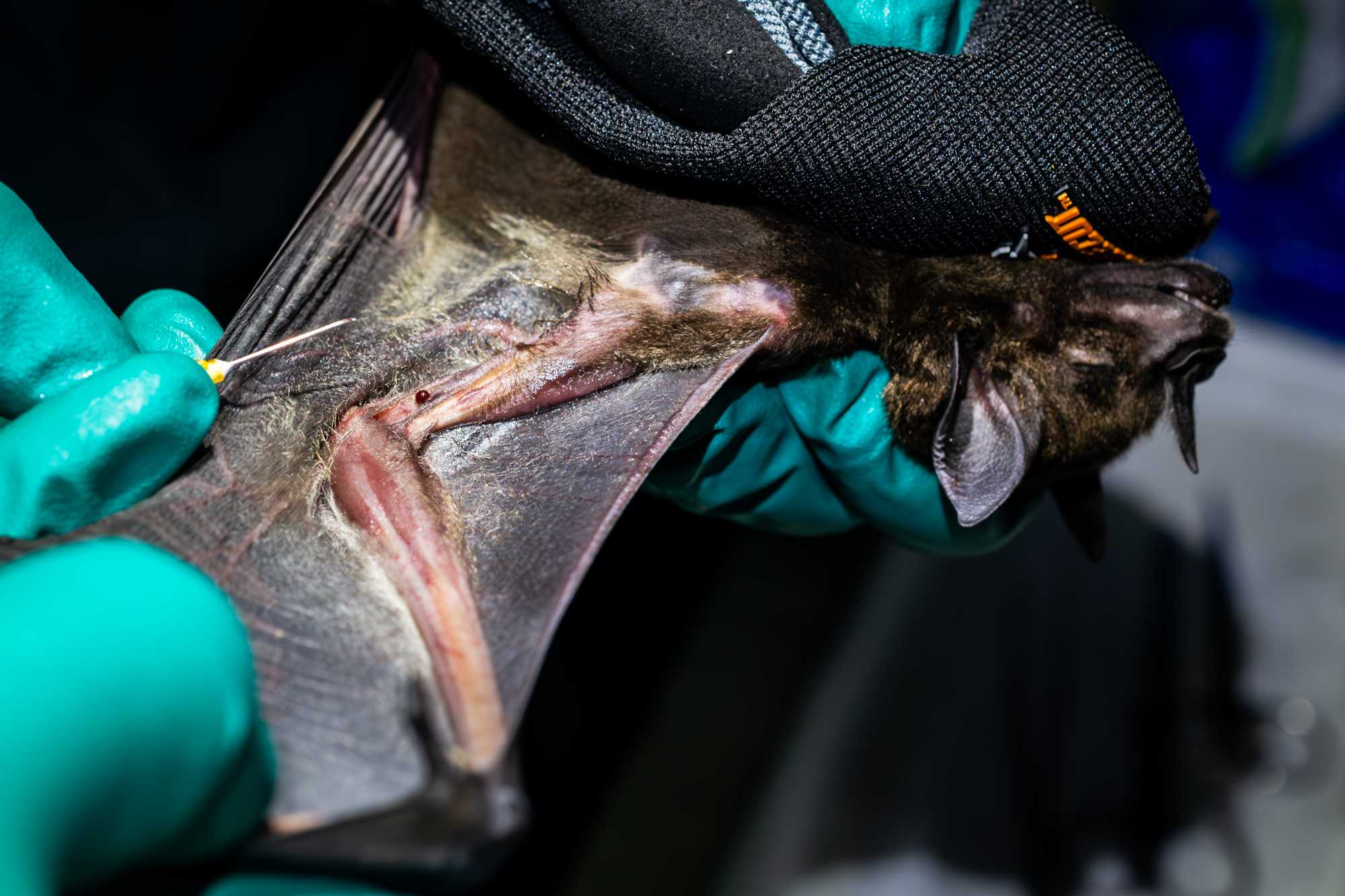
BLOOD
- The bat is manually restrained between the thumb and palm of the non-dominant hand and its wing extended until its fore and upper arm forms a 90° angle. The medial aspect of the wing should be facing the handler.
- The venipuncture site is prepared with an antiseptic swab and by applying a thin layer of vaseline with a clean cotton swab, in order to create a hydrophobic surface that allows the formation of a neat blood droplet.
- A 25 g needle is used to puncture the antebrachial vein.
- Touch the blood drop with the tip of a microhematocrit capillary tube. Bats must be bled with caution to maintain a ratio no greater than 6µL of blood per gram. Up to 0.6% body mass of volume of blood can be collected using microhematocrit capillary tubes*. (NOTE: some capillary tubes can hold up to 70μL of blood). From the capillary tube, blood is transferred into a 1.5mL tube containing lysis solution. If needed the capillary tube can be broken into the sample tube. Close the sample tube and shake to homogenize the blood sample.
- If the animal is sufficiently large, additional capillary tubes are collected for serum. Fill to ¾ and seal with clay on both sides so that it can be spun for serum collection. Keep sample cool in a labelled 15mL tube.
- Additional drops of blood are used for glucose and ketone reading and to create 2 blood smears.
- Use a cotton ball to put pressure on the venipuncture site and fold back the wing to hold it in place for about 2 minutes.
- Reopen the wing and check that the venipuncture site is no longer bleeding. If not bleeding, remove the cotton ball, otherwise apply light pressure until bleeding stops. Venipuncture site description
MEASUREMENTS
Measure all the body parts indicated in the processing sheet using a clean caliper.
Bat Measurements - ISL Peru
Inspect the wings and the body for injuries, measure, take pictures and record on the animal sheet.
ECTOPARASITE
Collect any ectoparasites that you might see, if necessary, cut some barbs where the ectoparasites might be hiding in between, and place them inside a 1.7 mL tube containing minimum of 70% ETOH.

Grab the animal by putting the dorsal aspect of the index finger in contact with the dorsum of the animal, and by holding the folded wings from the humerus. Use the thumb on one humerus and the middle finger on the other one.

Pull the animal out from the bag and place it with its ventrum on a thick leather glove to prevent your hand from being bitten (instead of a glove, the holding bag may be used if it has been folded onto itself to be sufficiently thick). With the thumb, hold the animal gently pressed against the glove, which is positioned on the palm of your hand.
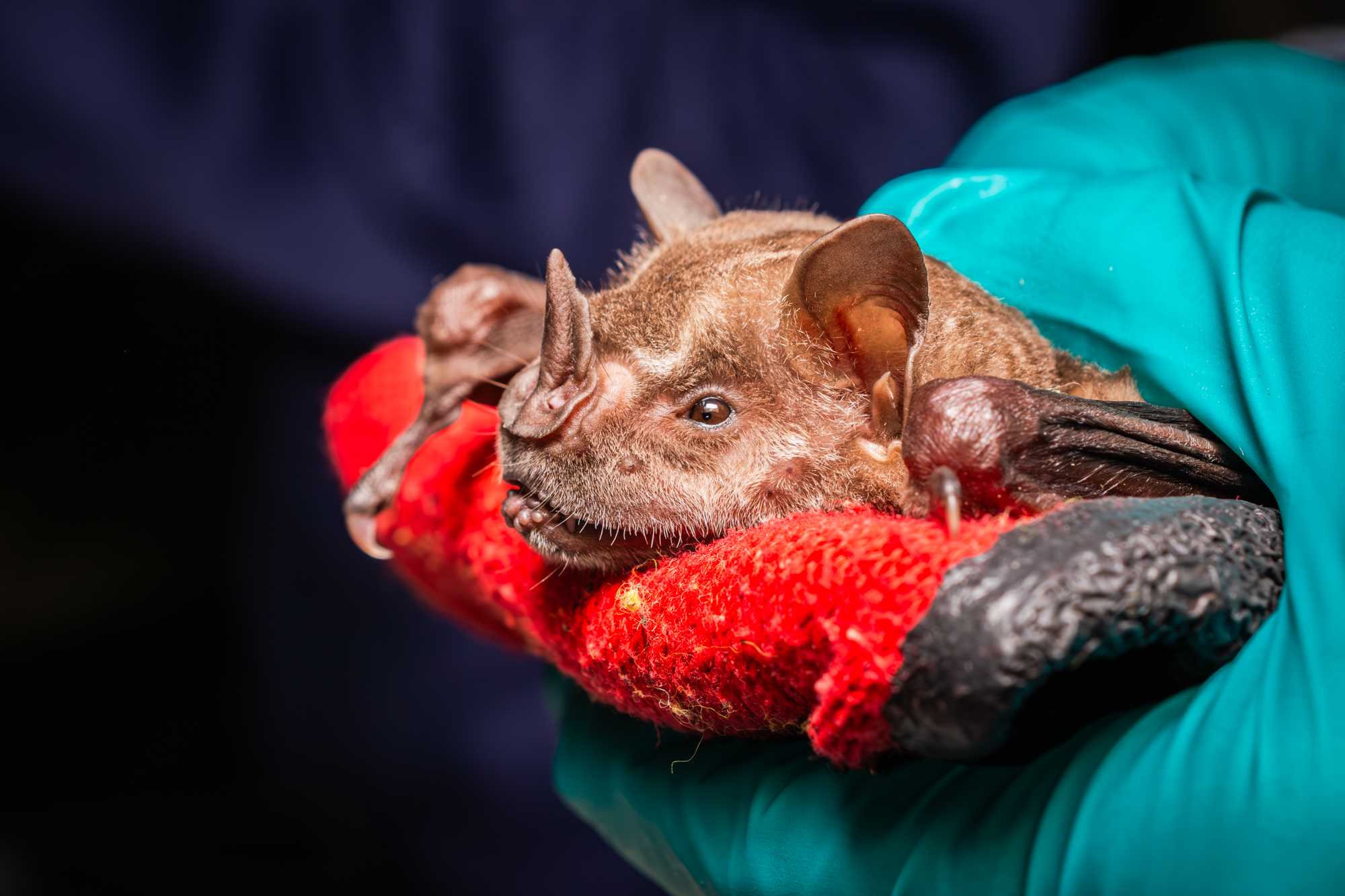
Use the dominant hand to grab the tools needed for the sampling or to move the wings of the animal.
Inspect the wings for possible biopsy-punch holes (usually on the right wing). If you find a punch hole that looks fresh, look along the dorusm for a patch of cut hair, which means that the animal is a recapture from the same trapping season. Taking pictures can help determine which individual it is.
In the case of recapture, the animal can be released straight away without undergoing SAMPLING.
FRUIT
Although rare, if a bat is found captured in the net with any fruit in its mouth, collect the sample into labeled zip-lock bag and record as an additional sample on the animal sheet

FECES
Check for FECES inside the holding bag and always collect feces opportunistically during the PROCESSING phase. Use clean forceps or a sterile swab to transfer feces into designated pre-labelled tubes or plastic zip-lock bags
Before collecting HAIR , take a picture of the dorsum of the bat if there is a morphological character present that could help identify the species.
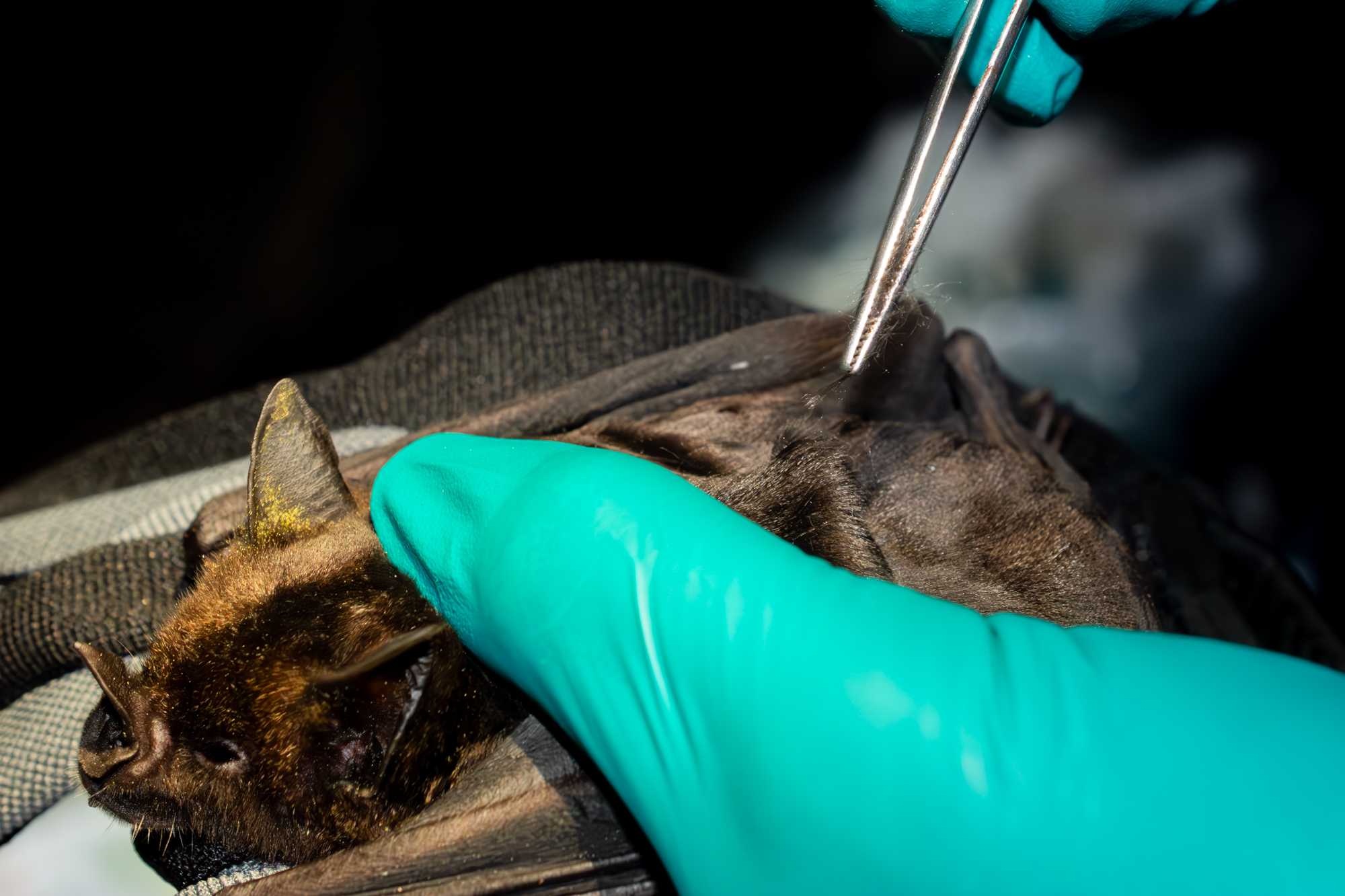
PLUCKED HAIR
From the dorsum, hold a few hairs with hemostatic forceps and pull gently in the direction of the hair, repeat the action several times to have a good amount with bulb collected. Place it inside a plastic bag labelled as: ”H-DNA” . Read clearly the serial number identifying the H-DNA bag for the voice recorder and for the data recorder.
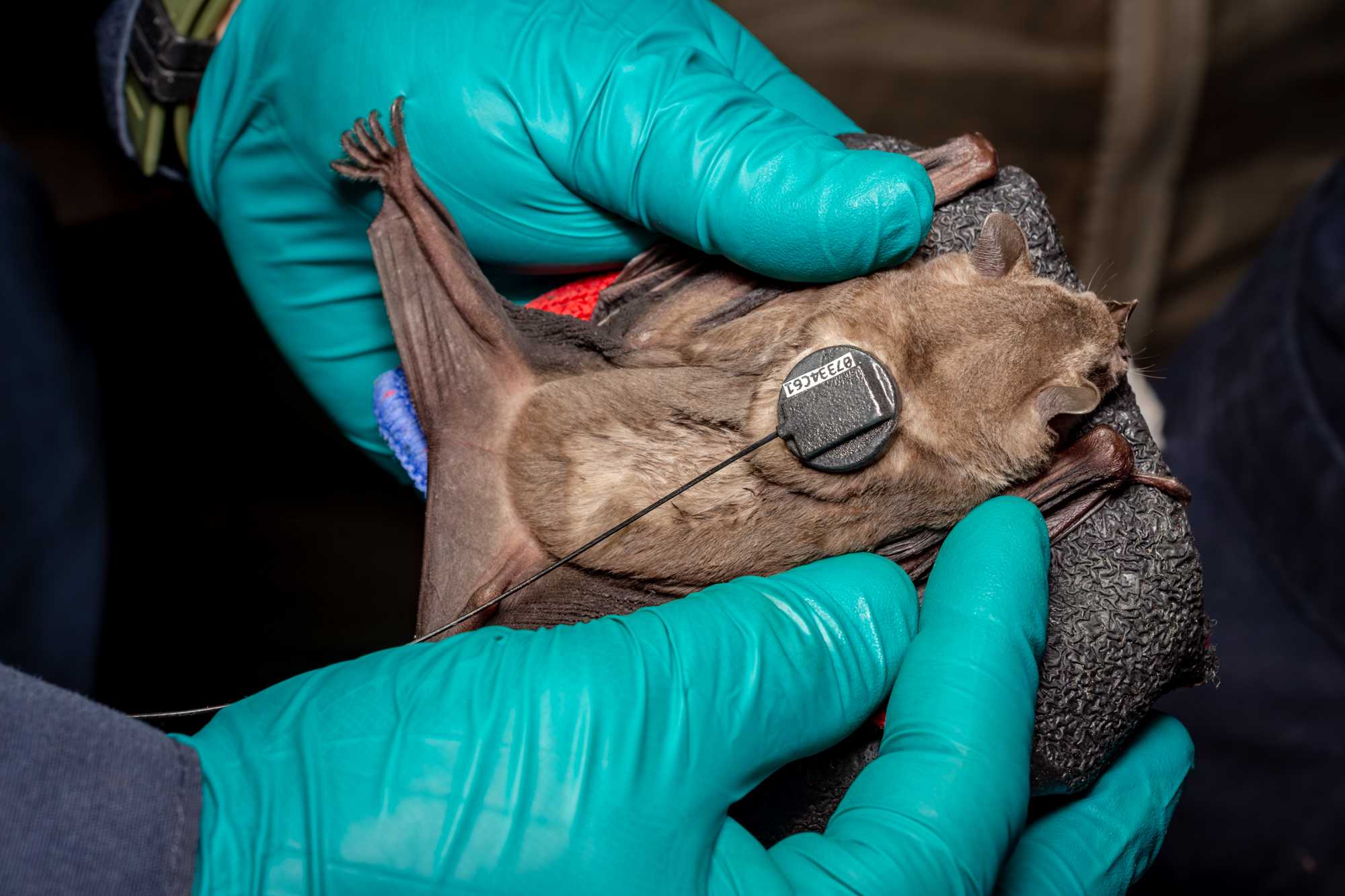
Tagging and Photos
zTracking & Life Tags
Prior to release and photos a subset of animals will be fitted with a UHF tracking tags (Cellular Tracking Technologies). Tags are temporarily glued to the dorsum (duration ~ 20days). Animals will only be considered for a tracking tag if their weight is >= 60g.
Transmitter attachment:
- Transmitters should be attached to the area between the shoulder blades so that the bats cannot use their hind feet to pull off the tag.
- For bats with shorter hair, transmitters will be attached without clipping hair, but for long haired bats its best to clip hair close to the skin. Do NOT full shave hair, some hair is provides a good matrix for glue binding.
- We choose Permatype Adhesive Surgical Cement as the adhesive of choice, based on tests on adhesion strength and time to tackiness (3-4mins).
- Apply a very thin layer of adhesive on the transmitter and on the bat. Let it stand for 5 mins until the glue bubbles
- Then press the transmitter to the bat applying a steady pressure for five more minutes
- To be certain the tag is fully fixed, do not release the bat immediately, but hold for 10 more minutes at a minimum.
- While holding, the handler and research assistant can begin to take photographs of the bat during that 10 minute time span.
- It is important to choose a large bat, unstressed by handling, and to provide nourishment (see above) during this process.
Permanent identification:
- Additionally, we intend that all bats will eventually receive a P-chip identification tag (https://p-chip.com/) which has been successfully used by SDZWA on the Pacific Pocket Mouse.
- P-Chips are micro transponder tags (500 μm x 500 μm x 100 μm) with photocells that are powered by a handheld laser wand connected to a computer or tablet and read through PharmaSeq’s p-Chip Reader software to emit a unique 9-digit signal (PharmaSeq Inc., Princeton, NJ).
- P-Chips are small, lightweight, and provide individual identification
- Tags come in an individually packaged sterile injector and are inserted subdermally in the uropatagium such that they are visible under the skin as a tiny black dot.
- Each p-Chip should be scanned with the handheld laser after tagging to record tag number.
P-Chip Placement
Clean the dorsal aspect of the right forearm of the bat using an alcohol swab. With the p-chip applicator, direct your needle with the bevel up parallel to the forearm.
Insert the bevel subcutaneously from proximal to distal and press the plunger to deliver the chip and safely remove the needle.
Check the insertion site for any superficial bleeding. Confirm the readability of the p-chip using the scanner.

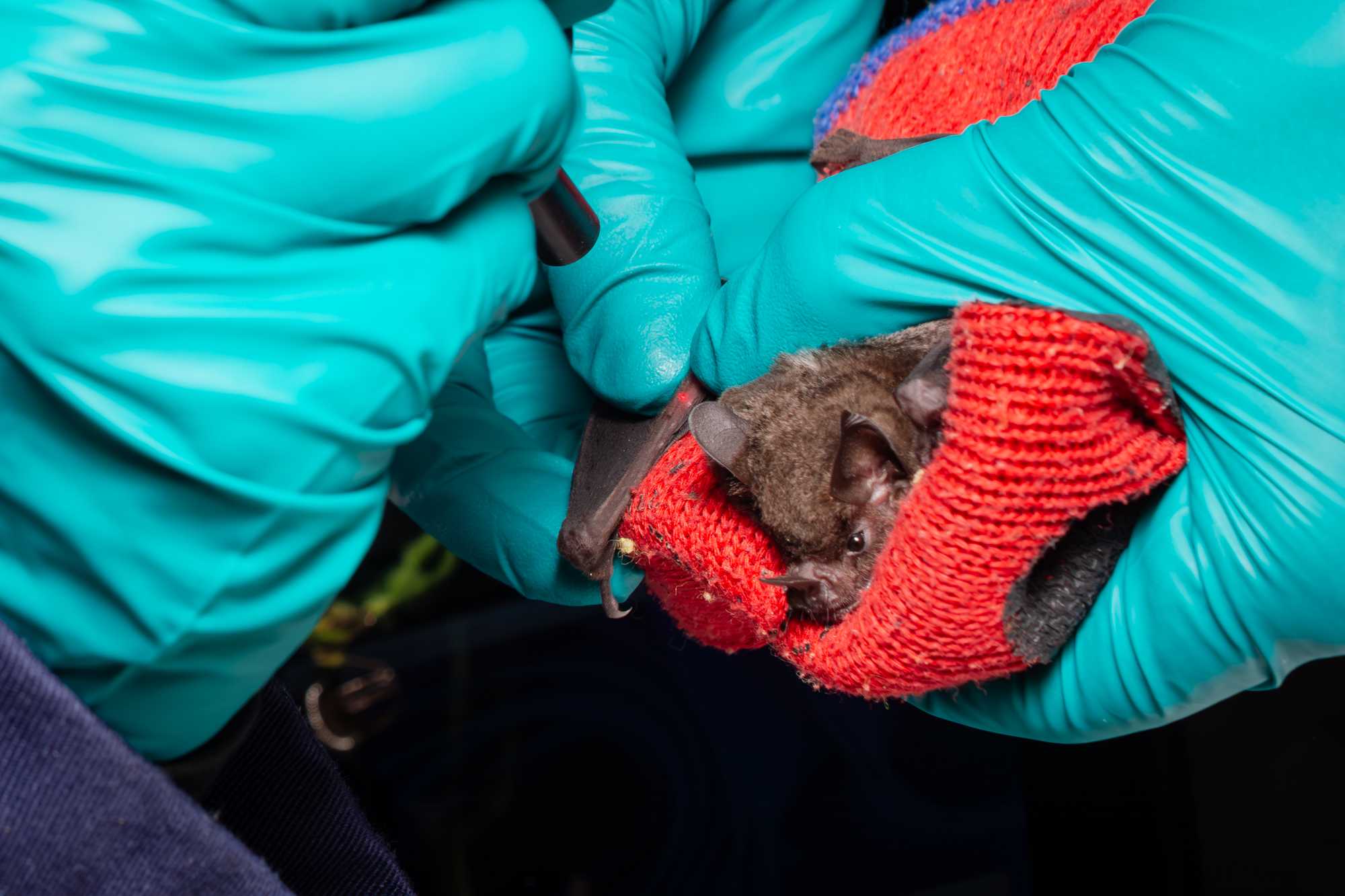
PHOTOS
While waiting for the tag to be secured on the bat, the handler and a research assistant can begin to photograph the pictures listed on the processing sheet.
After taking all the photos, the handler can check the tag and make sure it is secure.
Release
RELEASE
When PROCESSING is complete, the bat is taken outside and released by supporting it in an open hand with a protective glove, allowing the bat to push off and take flight.
The release can be recorded with a video and the RELEASE TIME should be reported on the processing sheet.
Record unusual behaviour of the animal at the time of the release and define it as IMMEDIATE or PROLONGED release.

Cleaning
CLEANING
Clean the surfaces that have been in contact with the animal or its secretions by:
- Spraying 10% bleach and then alcohol;
- Disinfect all the tools used during the processing with the sterilization solutions, as follows: leave submerged in 10% bleach for 5 minutes -> 1st rinse in distilled water -> 2nd rinse in distilled water -> submerge in 70% ethanol (begin this process immediately after using a tool, rather than at the end of processing each bat, to not lose time)
- Dry all tools and surfaces with with clean paper towels or sterile cotton.

Repeat this protocol for each consecutive bat following the capture order, unless instructed otherwise by the PI or team leader.
END SESSION
FIELD WRAP UP
Once all animals have been released the team should:
- End the Voice Recorder after stating the following: full date, location, number of bats processed, names of each bat team member.
- Securely close the nets. If nets accidentally open during the day or later in the night it will lead to animal injury or death
- Record "NET CLOSE" time
- Clean and pack materials used for processing
- Check, arrange and pack samples
- Return to base camp
.
FIX BLOOD SMEARS
- Arrange slides face-up on a clean surface and confirm that sample code is clearly visible, if not, trace over to make clear
- Identify a coplin jar with methanol that has not expired
- Open the jar and place smears inside (pairs can be placed back-to-back, MAKE SURE THAT SMEAR IS FACING OUTWARD). Quickly close jar again to prevent evaporation.
- Leave the smears in solution for 5 minutes
- Wearing a pair of gloves, remove each slide and place in an open slide box to dry overnight. Make sure to close the coplin jar as quickly as possible to preserve to the methanol
TEMPORARY SAMPLE STORAGE
Unless otherwise indicated by the PI, all other samples should be stored in the freezer until sample intake procedure the next day. NOTE, serum samples must be spun and extracted to a serum storage tube the same night and stored frozen.
END TASKS
Deposit all other materials in the laboratory prep area until morning noting the following instructions:
- Items that may be wet should be removed and placed somewhere to dry
- Materials that are dirty or came into contact with an animal should be in a secure bag or container, and marked as such.
- The voice recorder and data sheets should be stored in a safe place where they cannot be confused with that of other teams.
DAY AFTER TASKS
The following morning the team will:
Check any information on the processing sheet, and listen to the voice recording to fill in missing information on the data sheet with a differently colored ink pen (usually red).
Check that the laboratory team has all information they need for long-term sample storage
Re-supply and pack materials for the next capture session
Write a detailed narrative report of the capture session using the "trapping report template."
Gather pictures and videos from the session and sort them into designated folders on the project hard drive.
Scan the processing sheets and convert them into PDF files, named by the serial bat capture number. Attach them to the source record in fpi.insitulabs.app
Retrieve the recording from the voice recorder. Name the file using the following convention "YYYY-MM-DD_BATsession
Group all the files from point 17.3 to 17.6 into a unique folder named by the date and range of capture numbers used [yyyy-mm-dd_captures##-##]
Wash all the animal bags used during the capture with detergent and a disinfectant product, such as quaternary ammonium based solutions.