HEPES-Phosphate Medium, Suitable for Studies of Trace Element Nutrition in Photoautotrophic and Heterotrophic Auxenochlorella protothecoides.
Sabeeha Merchant, Dimitrios Camacho, Charles Perrino
Disclaimer
We would like to acknowledge Anne G. Glaesener for comments, suggestions, and revisions.
Abstract
This protocol describes a method for preparing a defined medium for phototrophic and heterotrophic Auxenochlorella protothecoides (UTEX 250). It is adapted from the commonly used TAP medium for Chlamydomonas reinhardtii (Goodenough, 2023, Chapter 11), with special attention to trace element supplementation (Kropat, Hong‐Hermesdorf, et al., 2011) and buffer composition. The Tris-acetate buffer is replaced with a HEPES buffer to control acidification of the growth medium as cells consume glucose. As a starting point, we determined the elemental composition of laboratory-grown Auxenochlorella protothecoides cells by ICP-MS/MS (Hui et al., 2022) and calculated how much of each essential element would be required in the growth medium to support growth of a culture with an initial density of 105 cells / mL to stationary phase (2.4 x 108cells / mL). The elemental composition of the medium before and after growth of cells to stationary phase was also measured to ensure that each element was provided in excess (2- to 5-fold) to accommodate variation in elemental quotas in response to the external conditions. All elements provided in the medium were measured except for H, C, N, O, and Cl. High purity chemicals ( > 99.999% pure with certificate of analysis indicating the level of contamination with other salts) are required for successful elemental deficiency and all glassware and plasticware are freshly washed with 6 M HCl (Quinn & Merchant, 1998).
Before start

Obtain only high purity chemicals which are usually labelled as “metals basis,” “extra pure,” or “ > 99.999%.” More importantly, check the “Specifications” tab of each chemical to verify that it has been assayed for the elements that you are interested in. Choose brands that have the lowest allowance for the elements that your lab is interested in. Contact the chemical’s manufacturers to get the certificate of analysis (CoA) for each chemical. Specify the lot number you have chosen so that the correct item is shipped.
When chemicals arrive, record each lot number and label the bottles with the date received, date opened, and quantity (e.g. 1 of 5). High purity trace metal grade chemicals typically have a higher cost than normal chemicals, so it is recommended that they be labeled and their use restricted for trace metal work. Avoid inserting spatulas into containers; instead, carefully shake or pour chemicals out. Chemicals brought out of the original container should not be returned into the container. Use plastic, non-metal, spatulas to handle chemicals.
Navigate to the supplier’s website and enter the lot number in the “Certificates” search tab. Download and review the certificate of analysis (CoA) of the specific batch. For example, you may find the certificates search tab for Millipore Sigma at this link: https://www.sigmaaldrich.com/US/en/documents-search?tab=coa You can view an example CoA for potassium dihydrogen phosphate by entering 1.05108 as the product number and B1642708 as the batch number. Record the values of each element you are interested in studying and calculate the potential concentration of contaminating metals expected in the final medium. See an example of this calculation for potentially up to 0.005 ppm of Cu contamination from potassium dihydrogen phosphate in equation 1. These estimates will provide insight into potential contamination, but ultimately, you should measure the final medium.

The final medium may have as much as 117picomolar (pM) of Cu from KH2PO4alone. Total potential Cu contamination in the medium will be the sum of potential Cu contamination from all chemical components. See table 2 for potential contamination from macronutrient stocks if 1 ppm of each metal is present in each chemical.

Use only ICP-MS grade ultrapure Milli-Q H2O, which can be sourced for example, from the Milli-Q® Advantage A10 Water Purification System (Model: Z00Q0V0T0). This system is internally outfitted with an A10 UV lamp (Cat# ZFA10UVM1), a Q-GARD® T2 pack filter (REF QGARDT2X1), and a Quantum® TIX Ultrapure Cartridge (REF QTUM0TIX1). An additional filtration step using a Q-POD® Element with a Quantum® ICP filter (REF QTUM00ICP) ensures ppt (parts per trillion) to sub ppt levels of trace elements. Before using the Milli-Q H2O, verify that the resistivity is 18.2 MΩ.cm at 25°C and the total organic carbon (TOC) reading is <10 ppb.
Steps
Wash all culture flasks, stock solution containers, and graduated cylinders with 6 M HCl
Dilute pure (certified ACS plus) 12Molarity (M) HCl to 6Molarity (M). To dilute 1L of 12Molarity (M) HCl to 2L of 6Molarity (M) HCl, add 1L of Milli-Q H2O to an empty 12Molarity (M) HCl bottle that has only previously contained unused 12Molarity (M) HCl (or use a dedicated mixing bottle). Slowly add 1L of 12Molarity (M) HCl to 1L of Milli-Q H2O. Always add acid to water and never add water to acid. The bottle may heat up as the acid is diluted, so let it cool before capping and mixing.
Do not use glass containers to store stock solutions as glass will leach metal contaminants. Use new (previously unused) wide-mouth polypropylene / translucent high-density polyethylene (HDPE) bottles. For macronutrient stock solutions, prepare five 1L bottles (Nalgene 2104-0032). For micronutrient stock solutions, prepare ten 250mL bottles (Nalgene 2104-0008) and one bottle for 2Molarity (M) NaOH. Keep the bottles capped as much as possible to avoid dust from entering the bottles.
For each bottle, fill with 100mL, 250mL, or 1L, of Milli-Q H2O to mark the fill line. This can be achieved by weighing the bottle with the appropriate amount of water. Repeat for all bottles.
Discard the Milli-Q H2O used in step 3 and add 250mL of fresh (unused) 6Molarity (M) HCl to one bottle. Cap bottle and swirl to wash the interior. Pour into the next bottle and repeat for all stock solution bottles. Rinse bottles with Milli-Q H2O at least 7 times.
To clean culture flasks, perform an initial 6Molarity (M)HCl wash by filling a 250mL culture flask with 100mL 6Molarity (M) HCl and swirl. Pour used HCl into the next flask and repeat for all flasks.
For the secondary wash, add 200mL of 6Molarity (M) HCl to each flask. Cover each flask with parafilm and leave in the fume hood for 24h 0m 0s or more. Ensure that the parafilm is properly adhered to the flasks so that they do not get sucked into the fume hood intake vents. HCl should never be left in the fume hoods uncovered. It is corrosive and may destroy the hoods.
Rinse each flask with Milli-Q H2O seven times (Quinn & Merchant, 1998). Rinse the exterior of the flasks as well so that there is no HCl residue. 6Molarity (M) HCl will corrode work surfaces. Leave cleaned flasks covered with parafilm so that no dust particles in the air (potentially with contaminating metals) enter the flasks.
Re-use or discard used HCl. The 6Molarity (M) HCl from the secondary rinse of culture flasks can be re-used up to 5 times or until a color change is observed (whichever comes first). HCl used for the first round of culture flask and stock solution bottle washing should not be reused and should be neutralized safely before discarding.
To neutralize 1L of 6Molarity (M) HCl, fill a 4L beaker with 1L water and put it in a secondary container within a fume hood. Slowly add 1L used 6Molarity (M) HCl to dilute to 3Molarity (M) HCl. Neutralize 2L of 3Molarity (M) HCl by adding NaHCO3 (Arm & Hammer pure baking soda) slowly, scoop by scoop (< 25g) until no foam is formed. Use a pH indicator strip to verify that the acid is safely neutralized (pH 7). Please refer to your institutional and municipal guidelines for disposing liquids with high concentrations of NaCl.
Make the macronutrient, thiamine, and buffer stock solutions on a bench near balances, rockers, and/or stirrers. Perform the filter sterilization (steps 13.4 and 14.3 ) in a sterile hood. Record the actual mass of chemicals added to each solution.
Make 1Molarity (M) HEPES Stock (1L)
Dissolve 238.3g HEPES in approximately 500mL Milli-Q H2O and fill to 1L. Mix well until dissolved.
Do not adjust pH.
Store at 4°C.
Make 2Molarity (M) NaOH Stock (100mL)
Add 8g of NaOH and fill to 100mL with Milli-Q H2O. Mix well until dissolved.
Store at Room temperature in a properly designated cabinet for strong bases.
Make 100× Phosphate Solution (1L).
Dissolve 153.77g of KH2PO4in approximately 600mL of Milli-Q H2O. Then add 38.15g of KOH and fill to 1L using Milli-Q H2O. Do not adjust the pH. Mix well until dissolved
Store at 4°C.
Make 40× Macronutrient Solution (N, Ca, Cl, Mg, and S) (1L).
Dissolve 1.5g of fresh anhydrous CaCl2 in approximately 300mL of Milli-Q H2O. Alternatively, use a liquid stock of CaCl2.
In a separate acid washed bottle, dissolve 11.55g MgSO4·7H2O and 16.05g NH4Cl in approximately 500mL of Milli-Q H2O completely.
Mix slowly with CaCl2 solution and fill to 1L with Milli-Q H2O. Mix well until dissolved.
Filter-sterilize the solution, using a 0.22 µm pore syringe filter with proper sterile technique in a sterile hood.
Store at 4°Cand only open the bottle in the sterile hood.
Make 2millimolar (mM) (1000×) thiamine stock solution.
Dissolve 675mg of thiamine hydrochloride in approximately 800mL of Milli-Q H2O.
Fill to 1L. Mix well until dissolved.
Filter-sterilize the solution using a 0.22 µm pore syringe filter in a sterile hood using proper sterile technique.
Store at 4°C. Open bottle in the sterile hood only.
Make the preliminary concentrated stock solutions of trace elements.
Make Pre 1 - 125millimolar (mM) Na2EDTA concentrate (250mL)
Dissolve 11.63g of Na2EDTA·2H2O in 180mL of Milli-Q H2O. Note pH needs to be adjusted to completely dissolve Na2EDTA. See next step.
Titrate to pH 8 with trace element grade2Molarity (M) NaOH solution (from step 11). Use 43mL – 47mL of 2Molarity (M) NaOH. Measure the pH by pipetting 30µL of solution onto a pH-indicator strip. Record the volume of 2Molarity (M) NaOH used.
Fill to 250mL with Milli-Q H2O. Mix well until dissolved.
Store at 4°C
Make Pre 2 - 285micromolar (µM)(NH4)6Mo7O24(250mL)
Dissolve 88mg of (NH4)6Mo7O24·4H2O in Milli-Q H2O and fill to 250mL. Mix well until dissolved.
Store at 4°C
Make Pre 3 - 1 mM Na2SeO3 (250mL)
Dissolve 43mg of Na2SeO3 in Milli-Q H2O and fill to 250mL. Mix well until dissolved.
Store at 4°C .
Make individual metal · EDTA stock solutions (1000×)
Make 25millimolar (mM) Na2EDTA (250mL).
Add 50mL of Pre 1 (125millimolar (mM) Na2EDTA concentrate from step 15).
Fill to 250mL with Milli-Q H2O. Mix well until dissolved.
Store at 4°C .
Make 28.5micromolar (µM) (NH4)6Mo7O24 (250mL).
Add 25mL of Pre 2 (285micromolar (µM) (NH4)6Mo7O24 from step 16).
Fill to 250mL with Milli-Q H2O. Mix well until dissolved.
Store at 4°C .
Make 0.1millimolar (mM) Na2SeO3 (250mL).
Add 25mL of Pre 3 (1millimolar (mM) Na2SeO3 from step 17).
Fill to 250mL with Milli-Q H2O. Mix well until dissolved.
Store at 4°C.
Make Zn·EDTA stock solution (250mL).
Add 22mL of Pre 1 (125millimolar (mM) Na2EDTA concentrate from step 15)
Add 720mg of ZnSO4·7H2O.
Fill to 250mL with Milli-Q H2O. Mix well until dissolved.
Store at 4°C.
Make Mn·EDTA stock solution (250mL).
Add 24mL of Pre 1 (125millimolar (mM) Na2EDTA concentrate from step 15).
Add 594mg of MnCl2·4H2O.
Fill to 250mL with Milli-Q H2O. Mix well until dissolved.
Store at 4°C.
Make Fe·EDTA stock solution (250mL) (does not use Pre1).
Combine 2.05g Na2EDTA·2H2O with 580mg of Na2CO3 and dissolve completely in 100mL of Milli-Q H2O
If using fresh anhydrous FeCl3 powder, add 811mg of anhydrous FeCl3 to the solution only after the previous two components are completely dissolved. Alternatively add trace metal grade FeCl3 liquid stock to a final concentration of 20millimolar (mM).
Fill to 250mL with Milli-Q H2O. Mix well until dissolved.
Store at 4°C.
Make Cu·EDTA stock solution (250mL).
Add 4mL of Pre 1 (25millimolar (mM) Na2EDTA concentrate from step 15).
Add 67mg of fresh anhydrous CuCl2 or add liquid stock solution (see note above) to a final concentration of 2millimolar (mM).
Fill to 250mL with Milli-Q H2O. Mix well until dissolved.
Store at 4°C.
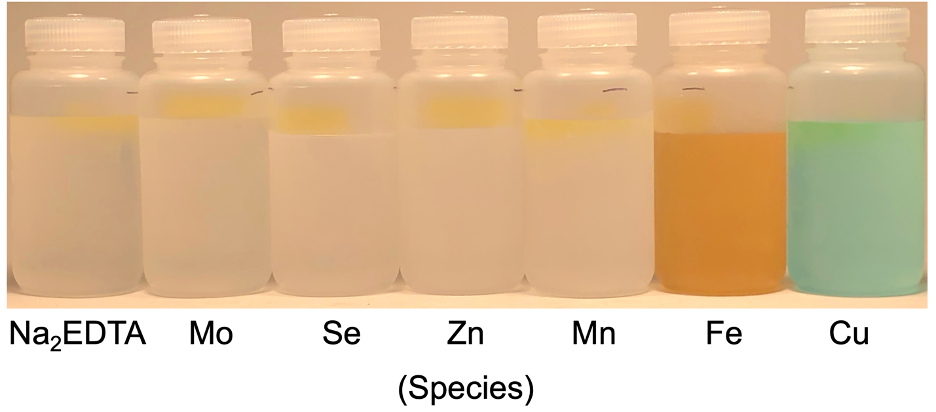
Making 1 L of HP medium
Close all stock bottles tightly and invert several times to mix.
Fill an acid washed 1L graduated cylinder to 700mL with ICP-MS grade Milli-Q H2O.
Add 10mL of 1Molarity (M) HEPES stock (from step 10).
Add 10mL of 100x phosphate buffer (from step 12).
Add 1mL of each trace element solution (from steps 18 to 24).
Use parafilm to tightly cover the graduated cylinder. Hold the parafilm in place with one hand and mix well by inverting the graduated cylinder at least 10 times. Alternatively, use an acid washed stir bar and an acid washed 1 L bottle to mix.
Add 2Molarity (M) trace metal grade NaOH (from step 11) to bring the pH to 7 – 7.5. Record the volume of 2 M NaOH used.
Measure the pH by pipetting 30µL onto the MQuant pH-indicator strips (non-bleeding pH 5.0 – 10.0, Supleco 1.09533.001).
For photoautotrophic growth, fill to 975mL. For growth with 2% glucose, fill with Milli-Q H2O to 935mL.
Use parafilm to tightly cover the graduated cylinder. Hold the parafilm in place with one hand and mix well by inverting the graduated cylinder at least 10 times. Alternatively, you may mix in an acid washed 1L bottle with a pre-labeled 975mL or 935mL fill line.
For photoautotrophic growth, aliquot 97.5mL into 250mL flasks using an acid washed 100mL graduated cylinder. For mixotrophic or heterotrophic growth, add 93.5mL.
Cover flasks with a double layer of 3 in. x 3 in. aluminum foil (Kirkland Signature Reynolds foodservice foil RK611 item 31680).
Put flasks in an autoclave bin and fill the bin with 200mL of H2O. Autoclave using the liquid setting. Cool to Room temperature.
Add 2.5mL of filter sterilized 40 x macronutrient (N, Ca, Cl, Mg, and S) solution (from step 13) to each 100mL flask or 25mL to 1L bottle using sterile technique in the sterile hood.
Add 100µL of filter sterilized 1000 x (2millimolar (mM) stock) thiamine (from step 14) to each 100mL flask in a sterile hood
For mixotrophic or heterotrophic growth, add 4mL of filter sterilized 50% glucose to each flask. For photoautotrophic growth, skip this step.
Store at Room temperature and use culture flasks within two weeks.
Please refer to your institutional and local rules and guidelines for proper and safe disposal of media and stock solutions.

