Gut-PFF Surgery
daniel.dautan daniel, Per Svenningsson
Abstract
Methods for injection of alpha-synuclein preformed fibrils in the gut in a mice.
Steps
Surgery Preparation
Prepare the needle.
We are using a 20µl Hamilton syringe that we polish the tip to a 20–30-degree angle with Thorlabs 30µm diamond sandpaper. Flush the syringe with ethanol and distilled water.
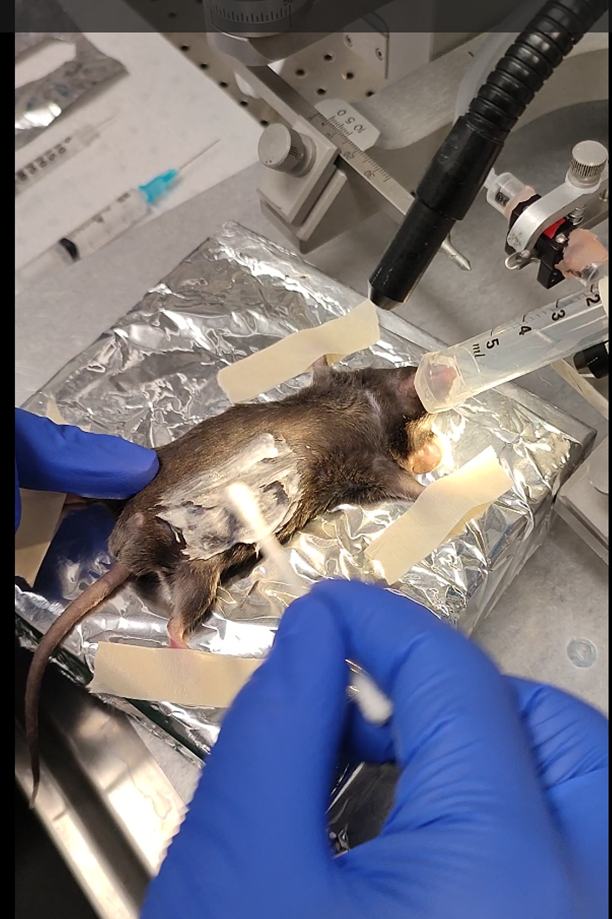
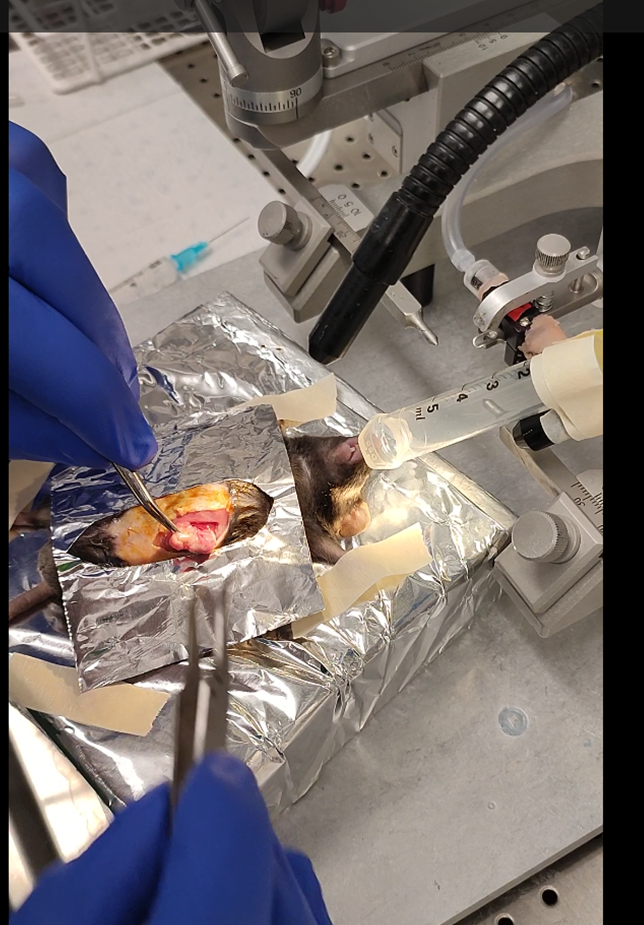
Prepare the mouse.
We recommend placing the mice on the back with a 10ml syringe connected to isoflurane (~1-2%) to anesthetize.
Use tape to spread the limbs as much as possible.
From this point, you will need to use a large blunt curve forceps and one sharp forceps also curved. Grabbing organs will be done using the blunt forceps, and grabbing fat will be done using the sharp.
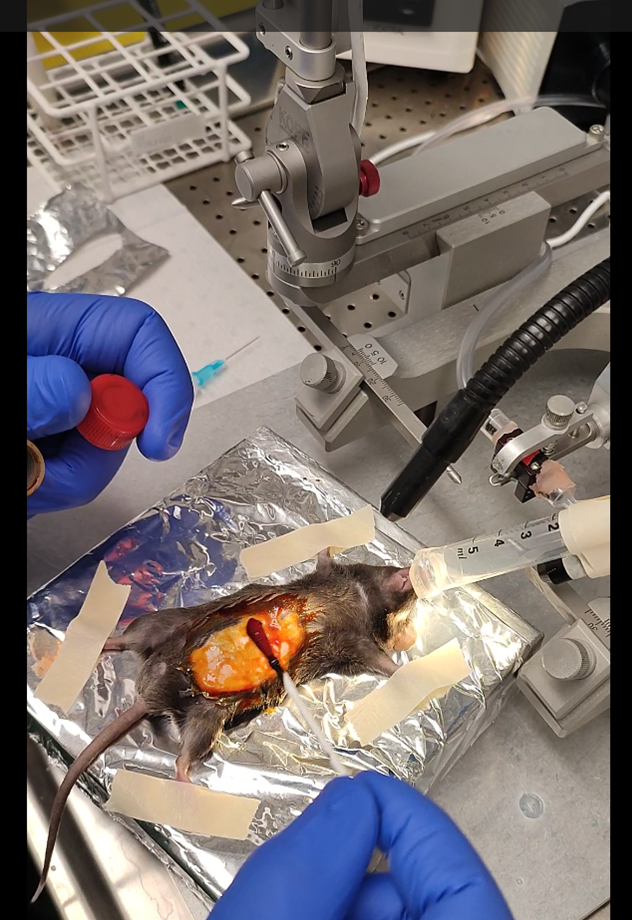
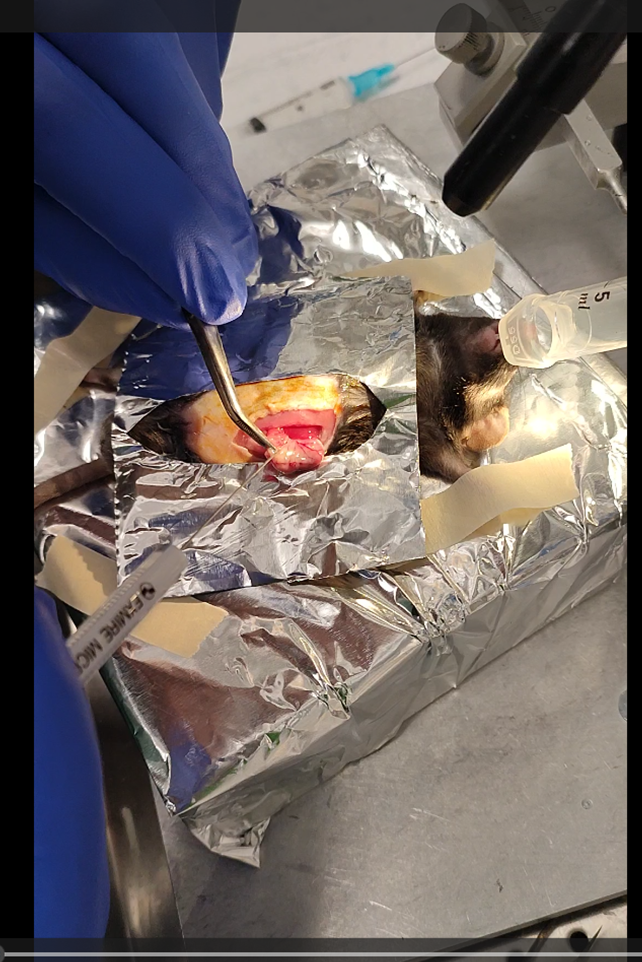
Take the stomach out of the cavity without damaging it. Do not worry the stomach is connected to a lot of tissue and can be pulled out easily. To avoid contamination, I recommend making a small surgical drape using aluminum foil that can be autoclaved.
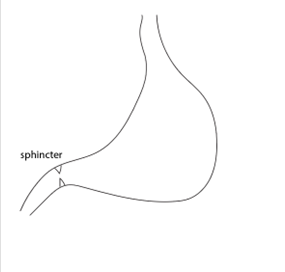
One needle penetration will be in the direction of the duodenum, and one in the direction of the wall. The first penetration follows the angle of the duodenum and allows one penetration to proceed to both injections.
After identifying the curvature of the stomach and the pyloric sphincter, load a 10 µl Hamilton syringe
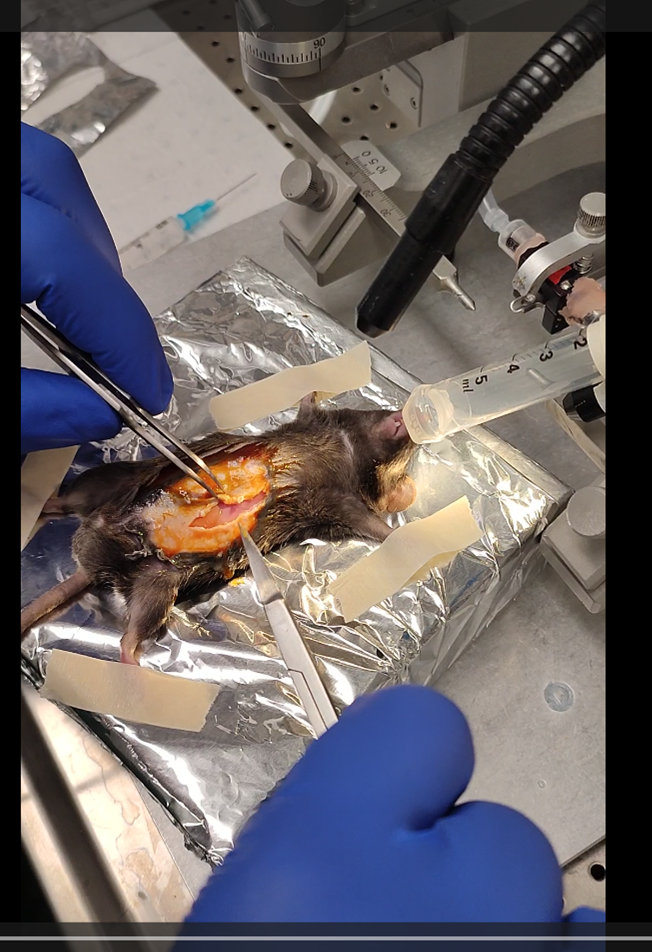
with 10 µl of αSyn PFF at a concentration of 2.5 µg/µl and insert along the pyloric canal. In a single penetration, inject twice (2.5 µl each) were administered along the lesser curvature of the stomach within the muscularis layer.


For the injection, I recommend using sharp forceps and penetrating the very superficial layers (muscular) of the wall. The needle must appear through the tissue by transparency as well as to have the needle as
parallel to the wall as possible.
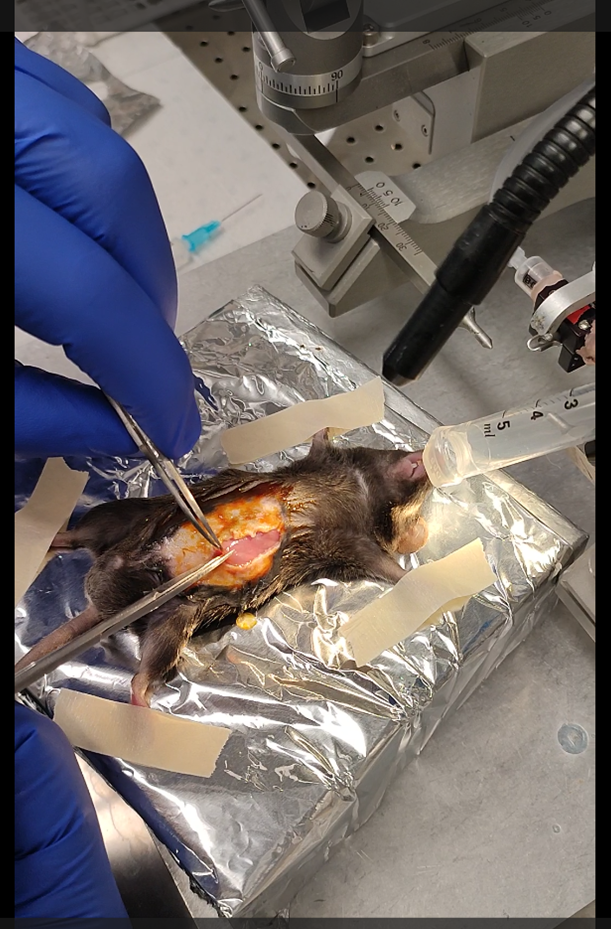
The second penetration will be around the main blood vessels of the internal wall. A second penetration,
perpendicular to the antrum, targets the muscularis layers of the duodenum adjacent to the sphincter. Deliver two injections (2.5 µl each) while slowly withdrawing the needle.
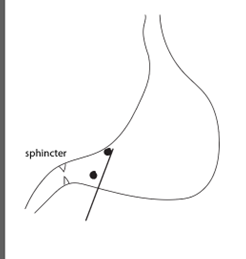
Based on the dilution of the PPF and the volume needed for the experiments, the 4 injections need to cover as much as possible the lower stomach and upper duodenum. To practice, I recommend adding a dye to the PFF to see the propagation of the liquid.
Following the closure of the muscles, flush with saline solution to avoid contamination.