‘From women for women’: A citizen science approach engaging women in the isolation and application of the vaginal health-associated bacterium Lactobacillus crispatus
Shardelice Illidge, Remco Kort, Rosanne Hertzberger
Abstract
A vaginal microbiome rich in Lactobacillus crispatus is associated with good reproductive and sexual health outcomes. Dysbiosis, indicated by a loss of Lactobacillus crispatus , is a risk factor for urogenital infections such as the clinical diagnosis of bacterial vaginosis (BV) or urinary tract infections. While many scientists explore probiotics using a conventional pharmaceutical approach, concerns about the accessibility and affordability prompt an investigation into a preventive approach using this naturally occurring bacterium. Our study is aimed at the exploration of a potential woman-friendly vaginal probiotic product by use of the naturally occurring bacterium Lactobacillus crispatus . Citizen scientists actively participated in a two-day practicum, successfully performing procedures with self-collected vaginal swabs. The practicum received a positive response from participants, who demonstrated notable engagement and enthusiasm. With expert guidance, participants without a laboratory background were able to successfully execute the assigned tasks. From the Dutch crispatus Citizen Science Collective of 48 women, 22 succeeded to isolate their own Lactobacillus crispatus strains, using a Loop-Mediated Isothermal Amplification (LAMP) protocol for identification. In addition, 48 metagenomes and 54 whole genomes from 22 individuals were sequenced for comparative analysis by an external company. This project effectively engaged a community of women into isolation of Lactobacillus crispatus strains from their vaginal microbiota followed by in vitro characterization experiments and a hackathon for the development of a probiotic product. Our citizen science approach opens up collaboration possibilities and new avenues for exploration in vaginal health, facilitating community involvement and the development of targeted intervention to enhance women’s well-being.
Before start
This protocol can be followed in a low-resource environment. All volunteers participating in the initiative must sign a consent form, ensuring they are both willing and legally competent to understand the implications of their involvement. It is paramount that privacy is upheld and guaranteed at all times, safeguarding the confidentiality of those involved.
Attachments
Steps
Practicals
We organised a two-day practical with two hour sessions each day. Upon registration, we shared additional information with potential donors about the importance and the reason for our project (Supplemental file 2: Appendix B. Participant information).
Participants received an online questionnaire (Supplemental file 3: Appendix C. Participation in the crispatus Study - Questionnaire, that focused on hormonal aspects, and a sampling kit at their home address containing:
Two sample collection tubes. One for metagenomic sampling (with DNA/RNA shield buffer), and one for microscopy and isolation (e-swab with Amies transport medium).
A swab for a pH self-test.
An information booklet that also served as a personal lab journal.
The contents of the sampling kit that a donor would receive are illustrated in Figure 1.

The women were advised to take the swabs a maximum of four hours before the start of the practical (Supplemental file 4: Appendix D. Section on Swab Sampling from the Lab Journal).
- Taking the swabs on location is also an option. We used a lactation room or bathrooms for this purpose.
- A short introduction is given about the purpose of the project and how we go about achieving our objective.
- Since the practical is taking place in a laboratory, basic safety lab instructions are given to the women.
The informed consent and licensing agreement were discussed and signed before starting the practical (Supplemental file 5: Appendix E. Consent form crispatus practical). The women were also informed that they could stop research participation at any time without having to give any reason for it.
Microscopy
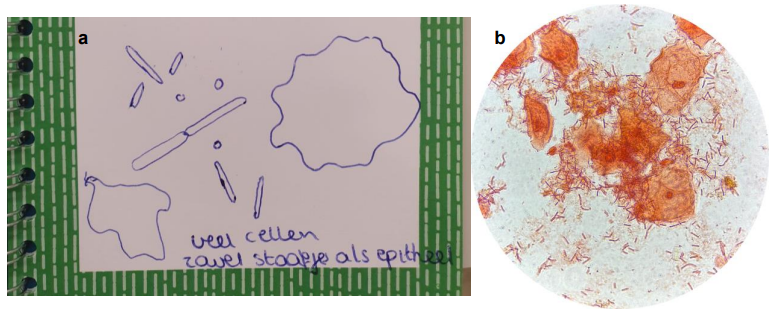
Citizen scientists fixed and stained a droplet from the e-swab collection tube on a microscope slide and were assisted to visualise their vaginal sample at 1000x magnification. Epithelial cells and lactobacilli were easily identified. Participants made descriptions of their samples in their lab journal and received a printed screenshot, as illustrated in Figure 2. See Supplemental file 6: Appendix F. Section on Microscopy from the Lab Journal, for the instructions provided to the donors.
Lactobacillus crispatus colony identification using a colorimetric test
For the identification of L. crispatus colonies we need to implement a microbial molecular technique that will be easy for the general public to carry out without the need for a microbiology training.
We used a L. crispatus -specific Loop-mediated isothermal amplification protocol (LAMP) as an alternative to colony PCR to allow participants to identify strains of L. crispatus [1].
Supplemental file 7: Appendix G. Section on Identification and Isolation from the Lab Journal, provide instructions to the donors and Supplemental file 8: Appendix H. Loop-mediated isothermal amplification protocol, for the protocol.
The LAMP is a DNA amplification method with high specificity and amplification efficiency. It allows the reaction to take place at a constant temperature, 65°C, within 0h 30m 0s.
It uses 4 primers and a DNA polymerase with strand displacement activity. The WarmStart® Colorimetric Master Mix (New England Biolabs, Ipswich, Massachusetts, United States) that is used in this protocol, has a low Tris buffer concentration at 8.
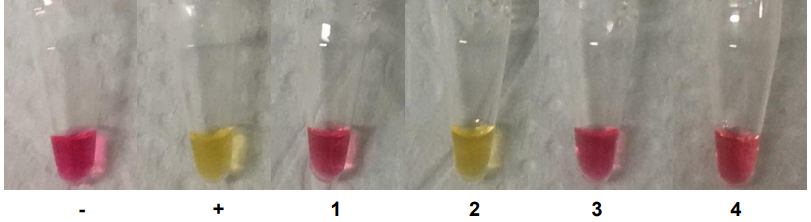
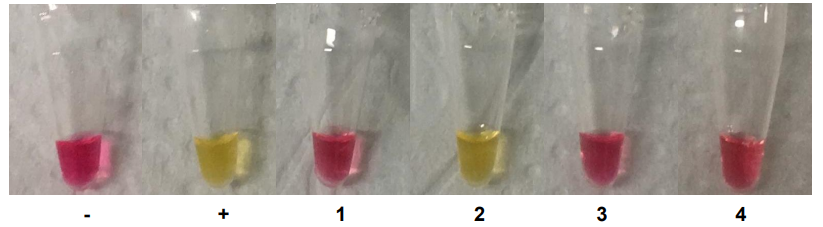
The mix contains a pH indicator, phenol red, that changes colour from red to yellow below a pH of 6.8, as shown in Figure 3 [2].
- This colour change occurs due to a drop in pH by protons that are released during the amplification process [3].
A previous study designed unique LAMP primers for L. crispatus and it showed that the LAMP test has a detection limit of 10 fg DNA [1].
- This was followed up by a dilution step in the protocol with
100µLMilli-Q to dilute any lactic acid associated with the colony and prevent false positives.
The LAMP test was validated using colonies of Lactobacillus plantarum , Lactobacillus delbrueckii sub sp. bulgaricus , Lactobacillus gasseri and Lactobacillus crispatus as illustrated in Figure 3.
Furthermore, we tested 134 colonies of the vaginal isolates obtained during the practical and compared the outcome of the LAMP test with a colony PCR using L. crispatus -specific primers [4].
- A total of 61 colonies showed a 145 bp band in the colony PCR analysis, matching 100% with the positive outcome of the LAMP test.
Isolation and cultivation
For L. crispatus isolation we used Tryptic Soy Broth (TSB) agar plates supplemented with 10% horse serum, 1g/L tween 80 and 10g/L glucose at5 adjusted it with 10% acetic acid as
previously described [5].
To ensure that each participant can successfully isolate L. crispatus colonies, despite substantial variation in bacterial load, each citizen scientist inoculated two TSB plates.
Inoculate the first plate with a large droplet application via a Pasteur Pipet. Spread the sample evenly over the surface using L-spreaders.
Inoculate the second plate with a small droplet using an inoculating loop for the streak plate method (See Supplemental file 9: Appendix I. Section on Sample Plating from the Lab Journal, for the instructions provided to the donors).
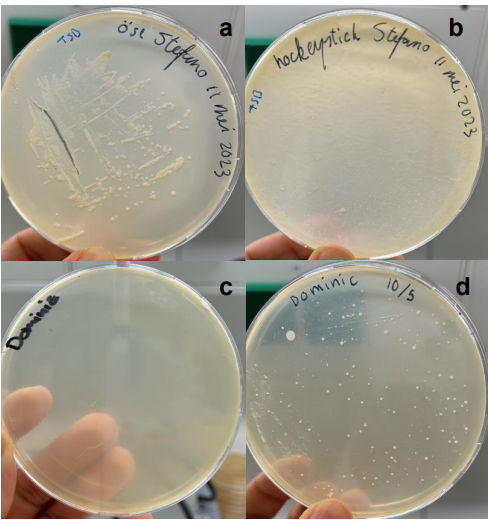
- Figure 4 illustrates the difference in bacterial growth between two citizen scientists on Tryptic Soy Agar.
Incubate the plates anaerobically at 37°C for 48h 0m 0s -72h 0m 0s using Oxoid™ AnaeroGen(Thermo Fischer Scientific, Waltham, Massachusetts, USA) system.
Select the colonies based on colony morphology which include being round, creamy white, with a raised central area, and having a slightly rough texture with irregular edges.
- Additionally, we used reference plates showing the morphology of L. crispatus along with other known lactobacilli including L. gasseri , L. plantarum and L. delbruecki subsp. bulgaricus .
Later on, we confirmed the identity of the selected colonies with the LAMP test.
Positive colonies are re-streaked on MRS plates.
- Alongside choosing their own pseudonym, participants are also able to name their L. crispatus isolate.

