Fluorescence-activated nuclei sorting (FANS) of purified neural cell populations from mouse cortex for multi-omic profiling
Stefania S Policicchio, Isabel Castanho, Barry Chioza, Joe Burrage, Jonathan Mill, Emma L Dempster, Jonathan P Davies
Abstract
Increased understanding of the functional complexity of the genome has led to growing recognition about the role of epigenetic/transcriptional variation in health and disease. Because epigenetic processes play a critical role in determining cell type-specific patterns of gene regulation it is important to consider cellular composition in regulatory genomic studies of heterogeneous tissue like the brain. Building on a previous protocol for isolating purified populations of nuclei from different cortical cell types from human post-mortem brain tissue, this protocol uses fluorescence-activated nuclei sorting (FANS) to isolate and profile nuclei from multiple different cell types from frozen mouse cortex. This protocol can be used to robustly purify populations of neuronal (NeuN+ve) and microglia (PU.1+ve) and other glial origin nuclei (NeuN-ve/PU.1-ve) from frozen mouse cortex tissue, with each sample yielding purified populations of nuclei amenable to simultaneous analysis of i) DNA modifications (via bisulfite sequencing/microarray), ii) histone modifications (via CUT&RUN-seq), iii) chromatin accessibility (via ATAC-seq), and iv) gene expression (via RNA-seq).
Steps
Nuclear prep for FACS separation (using NeuN, PU.1 and Hoechst)
In our hands the protocol below yields ~60,000 NeuN +ve (neuron enriched), ~5,000 PU.1 +ve (microglial enriched) and ~20,000 double negative (NeuN-ve/PU.1-ve; oligodendrocyte enriched) nuclei per ≤ 100 mg of frozen mouse cortex tissue. Recovery might vary from sample to sample due to high inter-sample variability and regional differences across cortical areas (i.e. dissection procedure, fat content of tissue sectioned, age).
Refer to Materials-Table 1 for details about the equipment required and to Materials-Table 2 for specifications of reagents required.
Solution and buffer preps
- Lysis Buffer (LB)
- Sucrose Solution (SS)
- Staining Buffer (SB)
Solutions should be kept at 4°C or On ice . Refer to Materials -Table 3 for recipes of solutions and buffers.
Nuclei isolation
1.Pre-cool the ultracentrifuge to 4°C before starting this stage of the protocol.
2.All buffers and the Dounce homogenisers should be pre-cooled on ice.
-
Add 1 mM DTT to the SS and LB according to the recipe (i.e. 17 µL per 50 mL of SS/LB)
-
Transfer 1 mL LB into each homogeniser
-
Add the dissected tissue sample into the homogeniser
-
Wait 3-5 minutes before douncing the tissue to allow the sample to defrost
To reduce heat caused by friction, the Dounce homogenisation step should be performed on ice with gentle strokes, and care should be taken to avoid foaming.
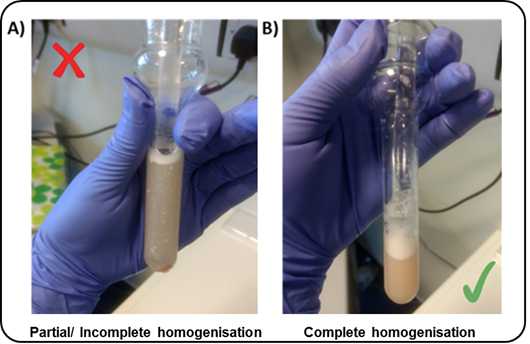
-
Transfer 8 mL SS (1.8M) to PA thin-walled ultracentrifuge tubes
-
Carefully overlay with tissue homogenate (1 mL per tube) - using a P1000 pipette, releasing slowly down the side of the tube
-
Add 1mL LB back into the homogeniser to rinse and recover as much as possible of the residual homogenate left behind
-
Recover volume from homogeniser and overlay it on homogenate phase
-
Balance opposite tubes by weight with 1x PBS using a fine microbalance
-
Perform ultracentrifugation for
0h 35m 0s(see Table 4 for centrifuge specification and conditions)
After Ultracentrifugation step
- Aspirate supernatant leaving 1-2 mL of the solution in the tube along with the pellet.
- Pour off any remaining supernatant, taking care not to dislodge the pellet (90-degree inclination of the tube). If the pellet is hard to see, it is okay to leave 100-200 µL solution in the ultracentrifuge tube
- Resuspend pellet in SB (1 mL), gently pipette up and down
- Let samples sit
On icefor0h 15m 0sat least (Blocking step) - Transfer volume into a new 1.5 mL tube
- Rinse out ultracentrifuge tubes in order to maximise nuclei collection by adding 1 extra mL of SB per tube, pipetting up and down several times, and transferring into a new 1.5 mL tube
- Washing step:
1.4x g,4°C,0h 0m 0sfor0h 5m 0s - Discard supernatant (pipetting off gently)
- Re-suspend each nuclei pellet in fresh SB (500 µL)
- If the sample was split then pool together resuspended pellets from the same sample (Final Volume = 1 mL)
- Add DNA dye (Hoechst, 2 µL/1 mL) and mix thoroughly by inversion.
- Pipette out 120 µL of nuclei solution for the Unstained Control (Hoechst dye only) and transfer to a new 1.5 mL tube
- Bring the volume up to 500 µL for the Unstained tube with fresh SB
- Replace the volume taken from the "Stained" tube with 120 µL of fresh SB (Final Volume = 1 mL)
Immunostaining
- Add the following three antibodies (Ab) to the "Stained" tube (1mL final volume):
- PE anti-PU.1 (1:100 dilution) – [10 µL Ab]
- Alexa488 anti-NeuN (1:1000) – [2 µL Ab ]
Refer to Table 5 for specifications of the antibodies used
-
Incubate tubes for
1h 30m 0son the rotor (speed=14 max) at4°Ckeeping the tubes in the dark -
Washing step:
1.4x g,4°C,0h 0m 0sfor0h 5m 0s(both "Stained" and "Unstained" tubes) -
Discard supernatant (by pipetting off)
-
Re-suspend in fresh SB (500 µL for the Unstained tube, 1 mL for the Stained tube - depending on pellet size)
Fluorescence-Activated Nuclei Sorting (FANS)
For machine start-up, CST and Accudrop calibrations refer to BD FACSAria III User’s Guide for guidance and troubleshooting. The following instructions describe FANS using BD FACSAria III. Other FACS platforms can be used but might require modifications to the protocol.
General Gating Parameters
For each sample, load stained and unstained tubes individually for data acquisition. A preliminary qualitative analysis of the data acquired is essential to select the appropriate gating strategy to maximise the nuclei capture while excluding unnecessary debris and to ensure an optimal signal/noise ratio.
Gating Parameters (X-axis: Y-axis):
FSC-A:SSC-A (Size, cell granularity or internal complexity)
SSC-W:SSC-A (to gate out doublets)
FSC-A:DAPI-A (to gate the single nuclei population)
DAPI-A:FITC-A (to gate NeuN stained nuclei)
DAPI-A:PE-A (to gate PU.1 stained nuclei)
FITC-A: PE-A (to visualize the distribution of the three detectable populations)
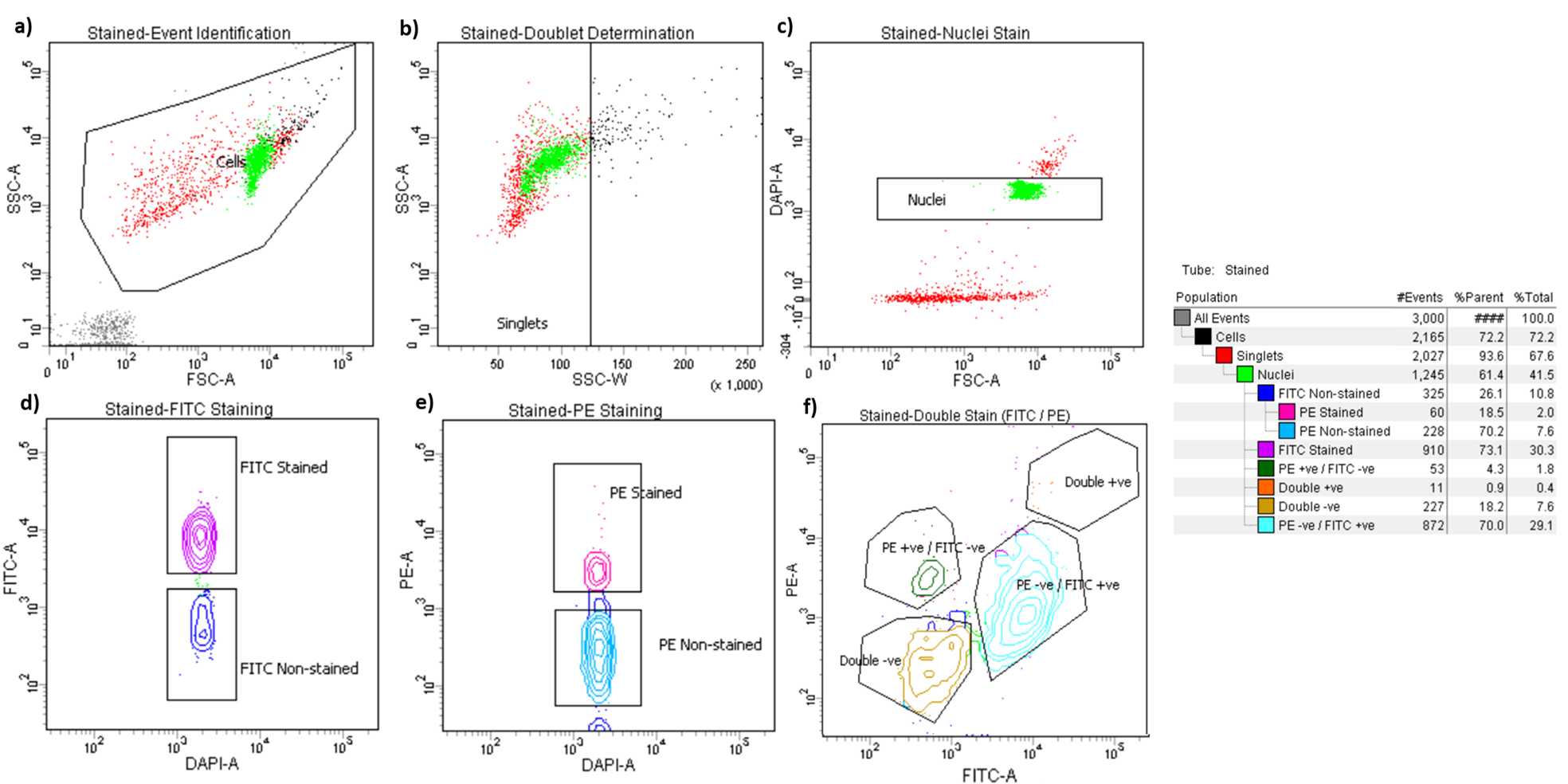
Data recording settings
In line with the experiment design, FSC, SSC, DAPI, FITC and PE are the parameters for which voltage values may need to be slightly adjusted due to experiment/ inter-sample variability.
It is advisable to set the threshold value between 200 and 500 during data recording. Moreover, in the acquisition dashboard tab, we recommend setting Events to Record ≤3,000, Event to display ≤5000 and Flow Rate = 1.0 (1,000 events per second) in order to increase the accuracy of signal detection.
The flow rate can be increased during sample collection to reduce the sort speed (ideally max events per second =1,500 for a 100-micron nozzle). However higher flow rates impact the data resolution and accuracy of events detection, and subsequent sorting of cellular fractions (see BD FACSAria III User’s Guide for details).
During analysis, recorded data is displayed in plots, while gates are used to define populations of interest for selection. Figure 3 shows a representative example of the two most common outcomes we often observe.
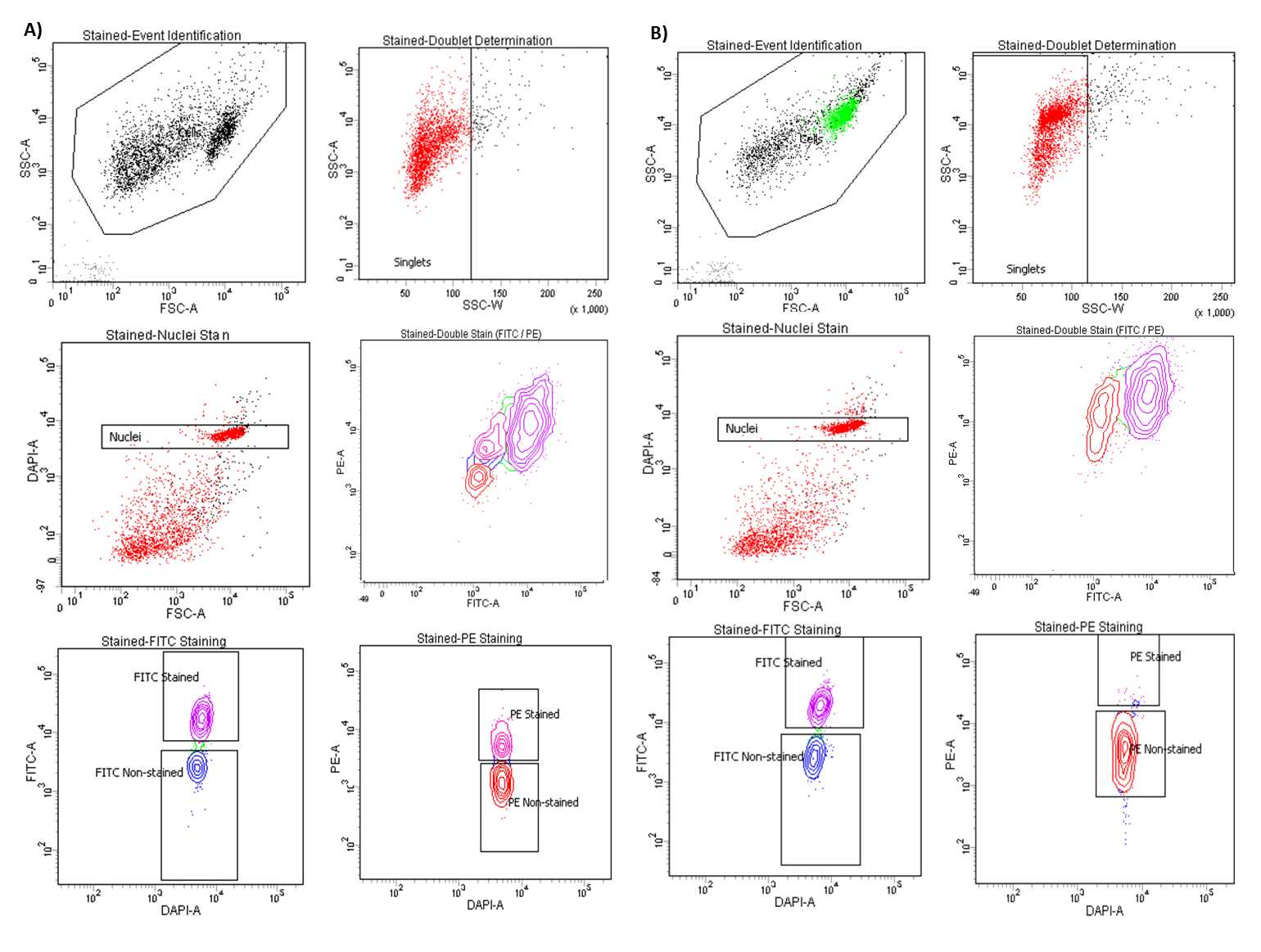
Sample Collection
-
LoBind Tubes (Eppendorf, Cat No:30108051) are required to collect nuclei (to maximise sample recovery of nucleic acids by significantly reducing sample–to–surface binding).
-
Nuclei are collected in 150 µL BAMBANKER (up to 1,000,000 nuclei) if collecting for chromatin or DNA assays. Nuclei are collected in Trizol LS Reagent (500 µL) if you are collecting for RNA.
-
Collected fractions can be used directly for downstream applications (e.g. DNA/RNA extraction, chromatin shearing) or frozen at -80 °C for long term storage. We recommend not to pellet nuclei down prior freezing to prevent nuclei loss due to nuclei bursting/ damage.
NoteNOTE - During collection, it is crucial to regularly pause the sorting to mix the two phases by inversions in order to preserve the integrity of resulting RNA preparations.For RNA extraction, LoBind Tubes should each contain 500µL of pre-chilled TRIzol™ LS Reagent (Fisher Scientific, Cat No: 11578616) prior to sorting. -
Keep samples on ice for the entire duration of the sorting
-
Lightly vortex sample tubes to make the mixture homogeneous (not clumped) before loading the tube into the FACS chamber
-
Load the UNSTAINED control tube into the chamber first and proceed with nuclei collection (for DNA, 200,000 events; for RNA, 300,000 events; for ATAC-seq 50,000 events). Refer to Materials - Figure S1 for the full range of applications sorted nuclei can be used.
-
Proceed by collecting STAINED tube by simultaneously sorting for NeuN and PU.1
General Recommendations for the user
For every new experiment we recommend performing the following steps:
-
When loading your tube into the FACS machine, run the unstained / IgG control sample first as this aids in setting the baseline parameters
-
Check your event rate in the Acquisition Dashboard window. If it is greater than 1500 evt/s turn down the “ flow rate ” or unload and dilute the sample further. If less than 100 evt/s, turn up the “ flow rate ” (don’t exceed a flow rate of 5.0 if possible, as the instrument is less focused and more inaccurate at higher flow rates)
-
In the Acquisition Dashboard window choose the appropriate “ stopping gate ” and “ storage gate ” (when working with nuclei, set as “Nuclei” and “All events” respectively)
-
Choose the range of “ events to record ” and “ events to display ” that best suits your purpose (≥ 5000 for both is advised)
-
Under the “ threshold ” tab in the Cytometer window, change the threshold (should be set for FSC) so that any small events in the bottom corner of the FSC vs SSC graph (caused by general cell debris and dust) are no longer shown. The threshold should not be set too high so that it causes an arbitrary, artificial cut-off through the left side of your population but not so low that small events caused by debris/dust are visible (ideally between a threshold 200-500).
-
Under the “ parameters ” tab in the Cytometer window, adjust the “ FSC ” and “ SSC ” values to get your population sitting in the centre of the FSC vs SSC graph (a re-adjustment of the “ threshold ” may be required at this point). It is essential to select “ restart ” each time any of the parameters are changed to update the events being displayed to ensure only events are recorded under the new settings.
-
Adjust or draw a new gate in the FSC vs SSC plot to encompass the population of interest.
-
Look in the scatter graph of SSC-A vs SSC-W (if you opened a blank experiment you will need to draw one). Right-click on the graph and check it is only displaying the events encompassed by your previous FSC vs SSC gate. Adjust or draw a gate for SSC-A vs SSC-W to encompass all the main population to the left of the graph and exclude outliers to the right (these are doublets and other cell debris clumps)
-
Under the “ parameters ” tab in the Cytometer window adjust parameters for the fluorochromes selected so the unstained / IgG control sample sits close to 0 for the fluorochrome on a graph of FSC vs fluorochrome.
-
Load the stained samples and check the stained population has a clear increase in signal for the fluorochrome in comparison to the unstained (signal should not exceed 104). Several minor re-adjustments of the fluorochrome’s “ parameters " may be necessary for the stained sample at this stage. If so, the unstained / IgG control must be reset and re-recorded.
NoteWARNING – Do not change parameter settings between samples you wish to compare, if you do you will need to re-record all samples using the changed parameters. -
Select the correct option for the collection device in the Sort Layout window ( we recommend “ 4-Way Purity ” for general collection)
-
Regularly check your “ Efficiency ” in the Sort Layout window value. Between 80-100% is ideal, 70% is acceptable if less than 70% either the sample is too concentrated or you are sorting a rare population. Although the “ flow rate ” in the Acquisition Dashboard window can be increased to make the sort quicker, faster flow rates are less efficient.
-
Check the “ Electronic abort rate ” (N° errors /sec) and “ Electronic abort count ” (Tot N° of errors) at the bottom of the Acquisition Dashboard window. These parameters measure potential miss-sorts (different from efficiency as efficiency measures undetermined drops which are directed to the “Waste” and therefore lost but do not contaminate).“ Electronic abort rate ” should be <1% of total events per second.
-
For long sorts, gate positions should be regularly monitored, especially for stained populations as fluorochromes lose intensity over time and the population can shift towards the unstained. Gates can be moved during long sorts to compensate.

