Enrichment of motile phototrophs by phototaxis
Usha F Lingappa, Sabeeha Merchant
Abstract
This protocol describes a method for enriching motile phototrophs from environmental samples by positive phototaxis, or the ability to move towards light. Samples are collected in the field using sterile equipment, and incubated in water and under lights to bloom phototrophs. Phototaxis is conducted in a sealed glass pasteur pipet filled with 0.15% agar, and wrapped in aluminum foil leaving only the tip exposed. Phototrophs must swim through the agar to reach the illuminated tip, separating them from the rest of the starting sample, and thereby generating an enriched fraction from which the phototrophs can be grown and isolated more easily than from a complex starting sample. This protocol is based on the method described in Sack et al , 1994.
Steps
Sample collection and preparation
Collect environmental samples (e.g. soil, sediment, water, etc. ) into sterile containers (e.g. Falcon tubes or Whirl-Pak bags), using sterile equipment (gloves, spatula/scoopula).
Encourage phototrophs to bloom by incubating samples under lights for 2-4 days.
Enrichment by phototaxis
Prepare 0.15% agar in minimal medium (e.g. Sueoka's High Salt Medium).
Add 0.15g agar to 100mL liquid medium.
Autoclave to sterilize and melt the agar.
Allow to cool to room temperature.
Prepare glass pipets for phototaxis.
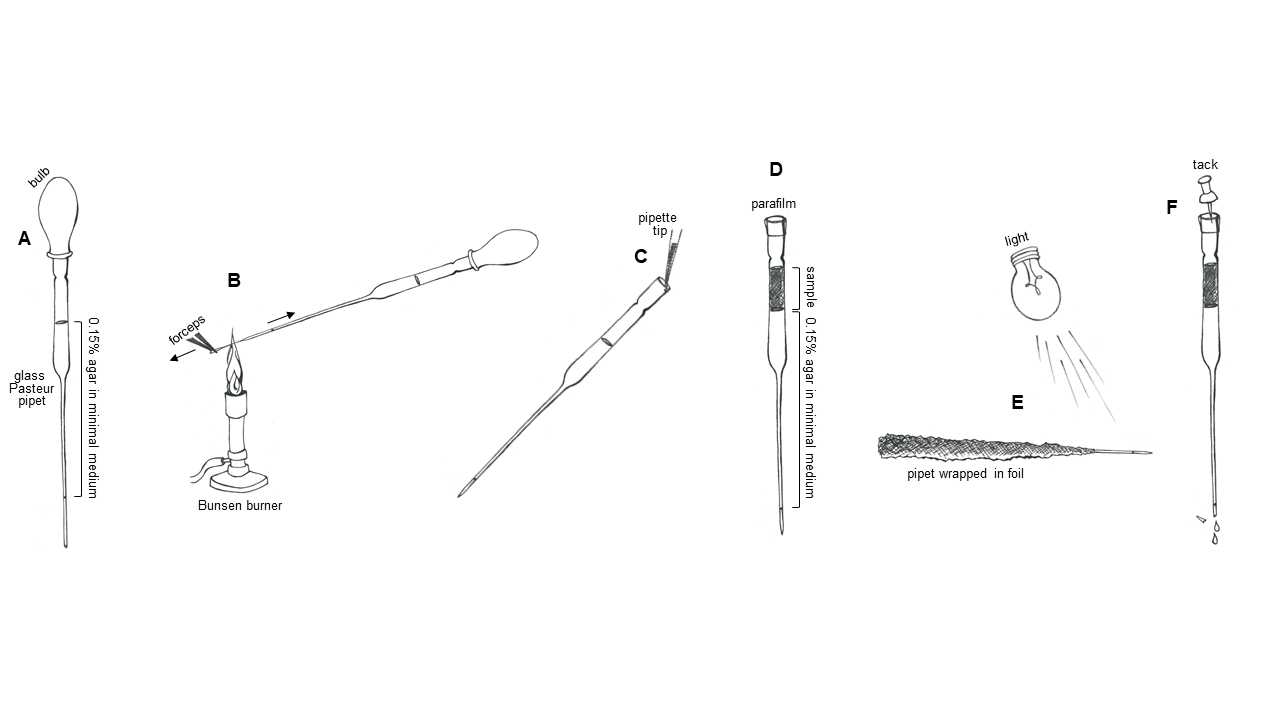
Fill pipets. Using a sterile bulb, fill a sterile 9 inch glass Pasteur pipet roughly half full with the 0.15% agar medium (Figure 1A). If your tip is full of liquid to the very end, let 1-2 drips to fall out to create ~2cm of empty space at the tip. This will help you to avoid generating bubbles by boiling the liquid when you put the pipet tip in the flame.
Seal pipets. In one hand, hold the pipet and bulb, maintaining pressure on the bulb to keep the liquid in the narrow part of the tip, allowing only ~2cm of empty space at the end. With the other hand, grasp the end of the pipet tip with forceps. Place the empty part of the pipet tip in the flame. As the glass melts, use the forceps to pull the tip away from the rest of the pipet, drawing out the glass into an increasingly narrow capillary (Figure 1B). Once the tip has detached, hold the melted end of the pipet in the flame for a few seconds longer to fully seal it. Allow the glass a few seconds to cool to room temperature, then gently test that you successfully sealed the end by applying additional pressure to the bulb. Once you have confirmed that the tip is sealed, remove the bulb.
Conduct phototaxis.
Load samples. Add 200-500μL liquid samples or extracts from solid samples onto the top of the prepared glass pipets (Figure 1C). Release the sample gently and against the pipet wall so that it layers neatly above the 0.15% agar medium.
Seal the top of the pipet with parafilm (Figure 1D).
Wrap the finished pipets with aluminum foil, so that the body of the pipet is kept in the dark and just ~2cm of the liquid in the tip is exposed (Figure 1E).
Incubate horizontally under lights for 4-24 hours, to allow motile phototrophs to swim to the illuminated tip (Figure 1E).
Harvest motile phototrophs.
Unwrap pipet.
Using forceps, break open the sealed glass tip.
Using a tack or needle, prick a hole in the parafilm sealing the top, to release the vacuum pressure holding the liquid in the pipet (Figure 1F).
Collect the first few drips of medium that release from the pipet tip (the medium that had been in the illuminated region).
Spread harvested material onto agar plates with a medium of choice for your target phototrophs (e.g. TAP for Chlamydomonas ), and incubate under lights until green colonies emerge.