Efficient transfection protocol of rainbow trout gills epithelial (RTgill-W1) cell line through nucleofection system.
Sebastián Escobar-Aguirre, Amanda Escorza, Matias Escobar-Aguirre
Abstract
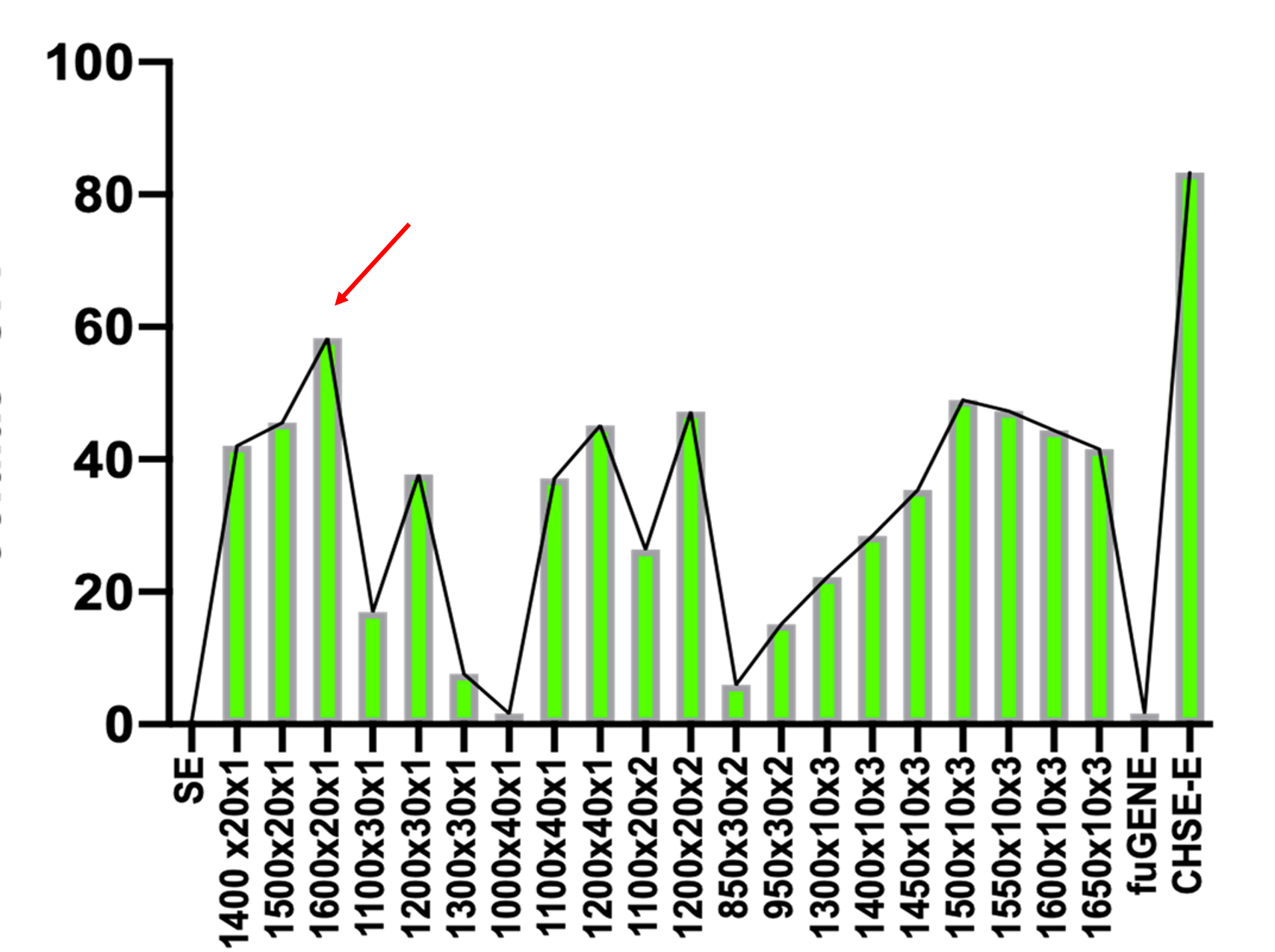
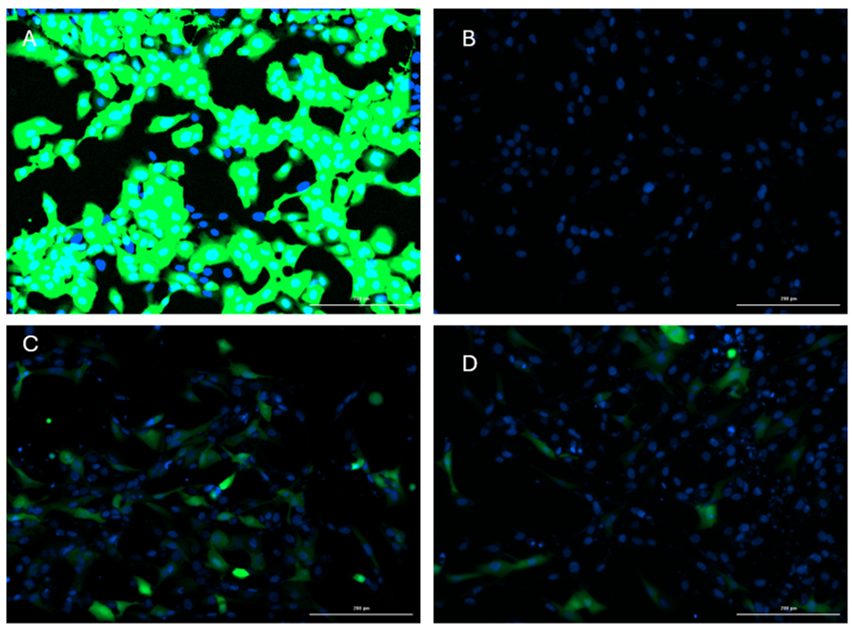
In this protocol, we optimize the experimental condition to express plasmid DNA in salmonid fish rainbow trout gills epithelial (RTgill-W1), using a nucleofection system. The principle of nucleofection is a combination of electrical parameters that ensures efficient DNA delivery into the nucleus, combined with low toxicity and high cell viability. Using the Neon Transfection System (InvitrogenTM MPK5000; code: 10431915) and the InvitrogenTM NeonTM Transfection System Kit 10 μl (brand: InvitrogenTM MPK1096; code: 10124334) we were able to express over 60% of GFP in RTgill W 1 line cells. These results were achieved after trying different optimization steps voltage (ranges between 850 - 1600 volts), pulse exposure (times 10 30 ms), and number of pulses, being the condition 1600 volts, 20 ms 1 pulse, and 1 μg μL of plasmid the most effective compared to the other conditions, presenting a higher percentage of GFP expression and lower mortality rate.
Before start
Cells were grown as a monolayer at 20 °C without CO 2 in Leibovitz L 15 medium (Cityva, USA) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) (Biological Industries, Israel) and maintained at 1 x antibiotics solution ().
The culture must be at 70 - 80% confluency to ensure the exponential growth phase of the cells.
The electroporations will be using 10µL tips and dispensed in 500µL of fresh media in a 48- well plate.
Steps
Cell preparation
Before electroporation, cells should be between about 70 - 90% confluent (i.e 6x10e6 cells/ml in a 175 cm2 flask).
Wash cells monolayer 2 times with 5mLand 1mL
Neutralize cells with growth medium 9mL into the flask.
Take an aliquot of trypsinized cell suspension, and register the viable total cell number by trypan blue 1:1 or similar.
From the total cell number (step 4), split 105 cells/well 5 cells/well (48-well plates) in a new tube (i.e 4 reactions = 4 wells, we would have 4×105 cells).
Centrifuge it 500x g,4°C to pellet the cells.
Remove the supernatant and wash with 5mL and repit step 6.
Remove all PBS and resuspend the cells in 10µL of Neon R buffer.
Prepare a 48-well plate with 500µL where the cells will be seeded post-electroporation.
NEON System Preparation
Prepare the NeonTM electroporation tubes with 3mL and place it inside the Neon Pipette Station.
Add cells to the tube containing 1 and gently mix. This plasmid encodes GFP (green fluorescent protein) as a reporter gene.
Insert the Neon tip into the Neon pipette (it is necessary to reach the second stop to open the clamp)
Electroporation
Aspirate the cell/plasmidial DNA mix with the Neon tip. BUBBLES INSIDE THE TIP SHOULD BE AVOIDED AS THESE AFFECT THE VOLTAGE TRANSFER.
Insert the Neon pipette together with the Neon tip into the pipette station vertically until a click is heard
Apply 1600 volts for 0h 20m 0s and 1 pulse
Carefully remove the Neon pipette from the station and immediately transfer the cells to a 48-well plate with medium WITHOUT ANTIBIOTIC and gently mix.
Culture in monolayer at 20 °C without CO2 in Leibovitz L 15 medium supplemented with 10% fetal bovine serum without antibiotic for 24 hours. Then change to fresh culture medium.
Determination of transfection efficiency
Quantify/Estimate the number of live cells after 72 hours post electroporation.
Determined transfection efficiency by measuring GFP fluorescence using the Cytation 5 platform from Agilent Technologies (excitation 469 emission 525).
It is recommended to use Hoechst staining (excitation 377 emission 447) to evaluate cell viability