Crude Membrane Fractionation of Cultured Cells
Dario R Alessi, Suzanne R Pfeffer, Asad Malik
Abstract
We present here a protocol for fractionating crude cellular extracts to prepare membrane and cytosol-enriched fractions and a nuclei-containing insoluble fraction from cultured cells. We deploy this protocol for determining the membrane versus cytosolic distribution of components from LRRK1 and LRRK2 signaling pathways.
Attachments
Steps
Crude Membrane Fractionation
5mL of ice-cold PBS. Immediately transfer the dishes to ice--this is best accomplished using wet paper towel-covered steel blocks resting On ice.
Add 5mL of ice-cold PBS and scrape the cells from the dish using a cell lifter (Sigma-Aldrich CLS3008, rubber tipped scraper, or equivalent) to ensure good yield; collect in a 15 ml tube.
Pellet intact cells by centrifugation at 100x g,0h 0m 0s for 0h 5m 0s at 4°C and aspirate supernatant.
Resuspend cells in 400µL of Buffer A by gentle pipetting.
Transfer to an 1.5ml Eppendorf tube and incubate On ice for 0h 15m 0s.
Add 100µL of cold Buffer B to the cell suspension.
Using a 25-gauge needle attached to a 1 ml syringe, break the cells by passing the cell suspension through the needle 25 times.
Centrifuge the cell suspension at 1000x g,0h 0m 0s for 0h 5m 0s at 4°C and collect the supernatant in a new 1.5ml Eppendorf tube.
Load the post-nuclear supernatant into thick-walled polycarbonate tubes, appropriate for ultracentrifugation in a table top ultracentrifuge. Ultracentrifuge at 150000x g,0h 0m 0s for 0h 20m 0s at 4°C.
Transfer the cytosolic fraction (supernatant) to a fresh Eppendorf tube On ice.
Wash the membrane fraction pellet will 500µL PBS thrice to remove any potential cytosolic contaminants.
Resuspend membrane pellet using 500µL of Buffer C using a pipet and incubate On ice for 0h 5m 0s 0h 20m 0s to allow detergent solubilization of membrane proteins.
Centrifuge membrane protein solution at 1000x g,0h 0m 0s for0h 5m 0s at 4°C to separate solubilized membrane proteins (supernatant) from insoluble membrane proteins (pellet).
Determine the protein concentration of cell lysates by Bradford assay according to the manufacturer’s instructions, performing measurements in triplicate.
4×SDS–PAGE sample buffer is added to samples containing 5µg of membrane protein or an equivalent volume of cytosolic protein, and heated at 37°C for 0h 10m 0s.
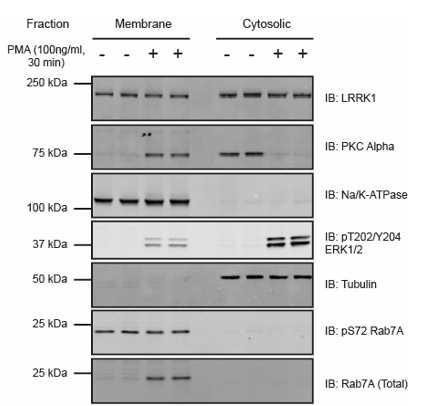
Analysis of fractionation products by quantitative immunoblotting analysis
The reaction products can be analysed by quantitative immunoblotting analysis (as described in dx.doi.org/10.17504/protocols.io.6qpvr68e3vmk/v1).
| A | B | C | D | E |
|---|---|---|---|---|
| Antibody Target | Company | Cat. number | Host species | Dilution |
| pS72 Rab7A | Abcam Inc. | ab302494 | Rabbit | 1:1000 |
| Rab7A (Total) | Sigma | R8779 | Mouse | 1:2000 |
| LRRK1 (total) (C-terminus) | MRC-PPU Reagents and Services, University of Dundee | S405C | Sheep | 1 g/ml |
| Tubulin | Cell Signaling Technologies | 2144 | Mouse | 1:5,000 |
| pT202/Y204 ERK1/2 | Cell Signaling Technologies | 9101 | Rabbit | 1:1000 |
| PKCα | Abcam Inc. | ab32376 | Mouse | 1:1000 |
| Na-K ATPase | Abcam Inc. | ab76020 | Rabbit | 1:10,000 |
Serum starve the cells for 16h 0m 0s and then treated ± Phorbol myristic acid (PMA) (100ng/ml) for 0h 30m 0s.
Following this, Perform the fractionation as described here and samples were subjected to immunoblot analysis with the indicated antibodies; the membranes were visualized using the Odyssey CLx scan Western Blot imaging system.