Community-based lifestyle intervention using targeted shared care approach versus usual care on pregnancy outcomes in Nigerian women with gestational diabetes mellitus (ALEVIATE GDM): study protocol for a randomized controlled trial
Victor M Oguoma, Ezekiel U Nwose, Ifeoma I Ulasi, Timothy C Skinner, Ekenechukwu E. Young, Euzebus C Ezugwu, Akintunde A Adeseye, Chinwuba K Ijoma, Nneka Iloanusi, Ifeyinwa Nnakenyi, Ikechukwu Obi, Sunday G Mba, Ejiofor V Ugwu
Abstract
Background: The purpose of the study is to evaluate the effectiveness of a targeted shared care approach in the treatment of GDM as well as to increase community awareness for early registration for antenatal care and those of healthcare workers about screening and management of GDM.
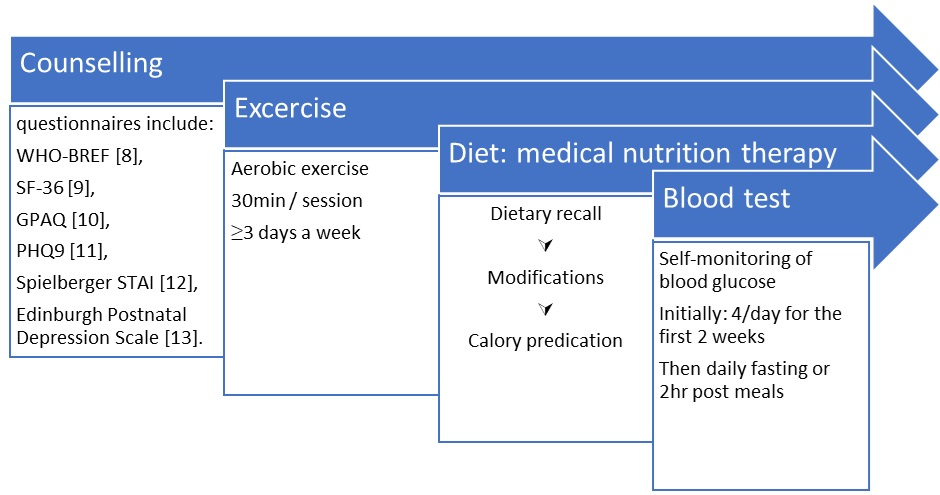
Methods: The randomized controlled trial will be conducted in the University of Nigeria Teaching Hospital (UNTH), Nigeria and extended to other primary, secondary, and tertiary health care facilities in South-East region and neighbouring communities of Nigeria. Participants will be followed up from baseline to 3 months post-delivery. Questionnaires (WHO-BREF, SF-36, GPAQ, PHQ9, Spielberger STAI, and Edinburgh Postnatal Depression Scale), Clinical (anthropometry, BP), ultrasonography (foetal parameters) and laboratory (urinalysis, glycaemia and lipids) data will be collected at recruitment and at intervals. Data on effects of education/awareness created and training of healthcare workers will be collected. Intervention would include usual care of general health talks on diet and exercise for control group. Shared care involving usual care plus individualized weight optimization counselling; dietary recall and self-monitoring of glucose. Primary outcomes include glycaemic control, birth weight ≥4000grams, registration for antenatal care and awareness of health workers. Secondary outcomes will include incident pregnancy-induced hypertension, perinatal complications, and community awareness.
Discussion : GDM screening and care at primary and secondary health facilities are almost non-existent. Hence the significance of this study would be in provide data on the utility of universal GDM screening as well as generate more representative up-to-date data for future planning and advocacy. Study findings shall be disseminated at conferences and peer-reviewed journals.
Trial Registration : Pan African Clinical Trial Registry (PACTR201804003328818), Registered April 2018, Protocol Identifier: BR2-R1-GDM1.
Steps
INTRODUCTION
BACKGROUND
There have been various studies on the prevalence of gestational diabetes mellitus in Nigeria, albeit using different criteria for diagnosis. Although guidance for management of GDM has been included in the Nigerian guidelines for management of DM [1], it is unclear the proportion of physicians and gynaecologists who adhere to these guidelines. Late registration of pregnant women for antenatal care has been reported in our environment [2]. This results in pregnant women with GDM being identified late in pregnancy, resulting in less desirable outcomes due to late intervention.
Universal testing is now being more widely advocated [3], although the major limitation is cost, which is an important consideration in LMICs like Nigeria. The health care structure in Nigeria including Enugu state is broadly divided into primary, secondary, and tertiary levels. The management of GDM is best done in tertiary health facilities, with an established chain of referral of women diagnosed with the condition. Yang et al had demonstrated improved outcomes in women with GDM who were randomized to a targeted shared care approach compared with usual care [4]. At present, screening for GDM at primary and secondary health facilities is hardly done or done haphazardly at best.
Interdisciplinary care is also not always available at all the levels of healthcare, being present mostly in tertiary health facilities. This study will aim to integrate care of women with GDM between the primary, secondary, and tertiary health facilities. The study will leverage on the existing healthcare structure in Enugu state, resulting in the training of healthcare workers and an improvement in the screening, identification, and management of women with GDM.
OBJECTIVES
The main aim of this study is to evaluate the effectiveness of a targeted shared-care (ShC) approach to treatment of GDM in comparison with usual-care (UsC) in a cohort of women with diagnosed GDM. The specific objectives include:
- To identify women with GDM in the rural, semi-urban and urban communities in Enugu State, Southeast Nigeria.
- To increase awareness of GDM among healthcare workers who manage antenatal women by 25%.
- increase community awareness for early registration for antenatal care by 25%.
- To increase regular antenatal attendance by 25%.
- To evaluate the effectiveness of a targeted shared-care (ShC) approach to treatment of GDM in comparison with usual-care (UsC) in a cohort of women with diagnosed GDM.
- To inculcate the practice of adequate follow-up of women with GDM post-confinement to avert/reduce future development of T2DM.
METHODS
Trial design and study setting
The study was conceptualized to be a randomized controlled trial with a single-blind design. A simple randomization procedure without replacement was adopted for assignment of consecutive eligible pregnant women with GDM to UsC or ShC. Allocation to each of the study sites will be uniform as all the study participants will be recruited as they become eligible.
Nigeria is made up of 6 geopolitical zones with a total of 36 states and the Federal Capital Territory. The zones are South-East, South-South, South-West, North-Central, North-East and North-West. The South-East comprises of 5 states (Enugu, Ebonyi, Imo, Abia and Anambra) with an estimated population of 30 million people.
Recruitment health facilities and population coverage
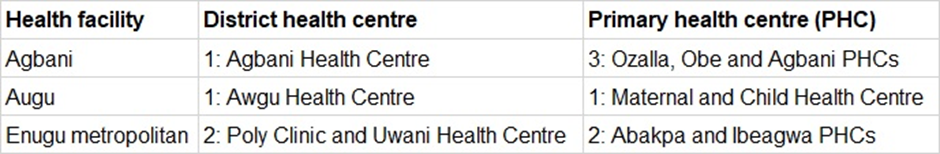
Enugu state, where the study will be conducted, has seven health districts namely Awgu, Agbani, Enugu-Ezike, Enugu Metropolitan, Isi-Uzo, Nsukka and Udi. The three health districts (Agbani, Awgu and Enugu Metropolitan) with a total of ten health facilities (Table 1), which were purposely chosen, serve a mix of rural, semi-urban and urban populations with a combined population of over 1,534,443 based on 2006 census increase by 2022 [5]. There are also two tertiary health facilities in the study area - the study centre, University of Nigeria Teaching Hospital, Ituku-Ozalla and Enugu State University Teaching Hospital, Enugu. Doctors and highly specialized health workers usually are in the SHCs and PHCs are mostly covered by community health workers (CHEWS) or in some cases lower-cadre nursing staff. Thus, participants will be recruited from the 12 sites.
Eligibility criteria for individual participating women
Pregnant women aged 18years old and above who fulfil the diagnostic criteria of GDM using the IADPSG criteria will be eligible for the study if they give informed written consent and do not have any of the exclusion criteria.
Exclusion criteria : Every consenting pregnant woman will undergo a fasting blood glucose or random blood glucose at first contact in the antenatal clinic if her pregnancy is below the gestational age of diagnosis of GDM. Women who have blood glucose levels in the diabetes range (FBG > 7.0mmol/l or RBG > 200mmol/l) will be considered to have pre-pregnancy diabetes mellitus [6], and excluded from the study. They will be referred to the obstetrician and endocrinologist for further treatment. Women who fulfill the diagnostic criteria for diabetes (fasting PG ≥ 7.0 mmol/L, 2-hour PG ≥ 11.1 mmol/L or HbA1c ≥ 6.5%), women with pre-existing/pregestational DM, multiple pregnancy, blood type incompatibility between mother and foetus – ABO type (Rhesus negative mothers will be pre-emptively excluded). Women with maternal diseases such as chronic hypertension, thyrotoxicosis, or those who use long-term medicines that could alter glucose metabolism will also be excluded.
Informed consent : Written consent will be obtained from the study participants by the research assistants and the investigators. Additional consent provisions for collection and use of participant data and biological specimens will include permission for collection of blood samples and ultrasound during the pregnancy.
The study process and interventions
Participant timeline
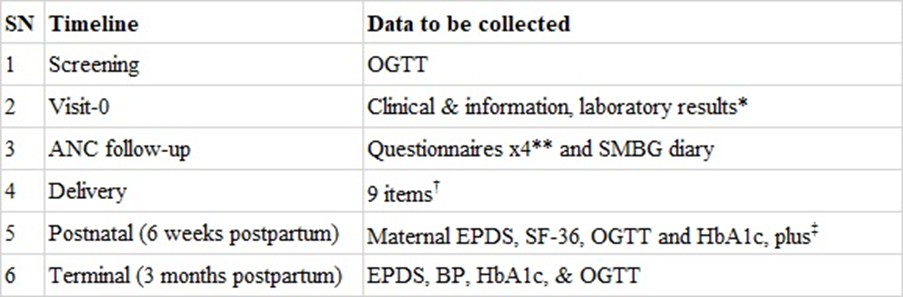
Screening Screening: At initial contact, every consenting pregnant woman attending the study site will have a FBG or RBG check to screen for diabetes mellitus if they are below 24 weeks GA. They will also complete the screening questionnaire. Those who do not fulfil the criteria for frank DM will be given an appointment to coincide with 24 - 28 weeks GA for OGTT.
Interventions
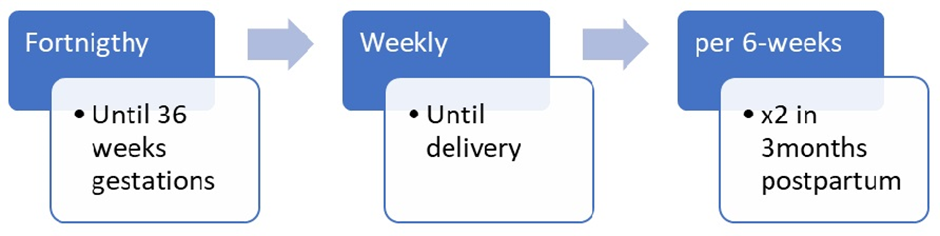
Intervention description: Intervention description : The intervention for the shared care arm will be based on a modified protocol of the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) study group [7]. All women in this group will be given individualized advice (dietary, physical activity and weight optimization counseling) and taught self-monitoring of blood glucose. They will receive individualized dietary counseling from a dietician at the study entry. They will also be counselled to engage in daily exercise for at least 30 minutes. The shared care intervention will thus consist of an individualized multidisciplinary approach to care as opposed to the usual care approach which consists of general dietary and health care advice. All women (UsC and ShC) involved in the study will be part of the usual care advice sessions; subsequently, to ensure the integrity of the masking, all participants will have private sessions. During these private sessions (Fig 1), the participants in the ShC arm will have counselling, blood glucose self-monitoring, and lifestyle interventions (Fig 2).
Criteria for discontinuing or modifying allocated interventions: Criteria for discontinuing or modifying allocated interventions :
To preserve the integrity of the randomization, women in each study arm will be given follow-up appointments on different days of the week. Intervention will be discontinued in women who withdraw consent or fail to attend > 40% of counselling or appointment sessions.
Oversight and monitoring: Composition of the coordinating centre and trial steering committee Composition of the coordinating centre and trial steering committee
The overall day to day coordination of the study will be done by the principal investigator and the co-investigators. There will be a trained research coordinator, who will be in charge of monitoring recruitment at all the study sites and the activities of the other research assistants and nurses. There will also be a multidisciplinary team consisting of an endocrinologist, obstetrician, sonographer, diabetes educator, dietician and laboratory technician who will be involved in monitoring and follow up of the recruited patients.
The study PI, co-investigators and multidisciplinary team will make up the trial steering committee. They will meet at least quarterly to evaluate the trial progress. The International Diabetes Federation will provide oversight function with the help of the grant coordinator and the study mentor. They will oversee the study by reviewing monthly study reports and also organizing teleconferences and physical meeting as needed.
Study Outcomes and management
Primary outcomes
There will be multiple primary endpoints for the study. The primary outcomes will be good glycaemic control defined as SMBG levels of FBG < 5.3mmol/l, 2hours postprandial < 7.0mmol/L and HbA1C < 6.2% as well as a reduction in fetal macrosomia defined as birth weight ≥4000 grams and/or the delivery of large for gestational age baby using the Nigerian local reference estimated from standard birth weights derived all hospital deliveries. This will be extracted from hospital records. Reduced fetal macrosomia is expected to result in better pregnancy outcome in women with GDM by a reduction in perinatal mortality and foetal abnormalities. It is also known that children born to mothers with GDM are at increased risk of future diabetes in adulthood [14]. An improvement in pregnancy outcome may likely reduce this risk. This is of public health significance as it is likely to impact positively on the future prevalence of diabetes in the community.
Secondary Outcomes
The secondary outcome for the study will be a reduced incidence of pregnancy-induced hypertension (PIH), reduced perinatal complications; such as the presence of shoulder dystocia and foetal abnormalities. Other measured outcomes will include reduced pregnancy and postnatal weight gain, reduced persistence of hyperglycaemia at the study termination, improved wellbeing and quality of life of the women and increased community knowledge about GDM.
These will contribute to improved pregnancy outcomes and maternal and child health indices in the community. Others include increased registration for antenatal care, increased regular attendance for antenatal care and improved proficiency of health workers to screen for, detect and manage GDM. A general improvement in accessing antenatal care in the community will also lead to better future pregnancy outcomes in the community, which can be sustained with ongoing future public health enlightenment campaigns.
The project will increase the awareness of diabetes and emphasize lifestyle measures to prevent it in the communities. It will also strengthen the identification and care of women who have GDM and generally improve antenatal care. The adoption of the shared care lifestyle intervention will likely improve both maternal and foetal outcomes both immediate and long-term, ultimately, saving lives and reducing the cost of healthcare. Proper lifestyle measures and follow-up in the women will also reduce their risk of future diabetes and contribute to an overall reduction in the incidence of diabetes in the community.
Sample size
Assuming 20% prevalence of macrosomia in the control group [15], and 11% absolute risk reduction in the treatment group, we would expect a sample size of 400 (200 per group) at 80% power and alpha level of 0.05 after taking into account 20% withdrawal or loss to follow up. If we also assume 17% prevalence of PIH in the control group [16], and 11% absolute risk reduction in the treatment group, we would expect a sample size of 438 (219 per group) at 90% power and alpha level of 0.05 after taking into account 20% withdrawal rate. Given these two scenarios, we would use a sample size of 438 (219 per group).
Recruitment will be consecutive and will continue until the sample size for the study is achieved. Community campaigns will be done from time to time to create continued awareness for the study in the communities to increase antenatal registration and subsequent recruitment yield.
Statistical methods
Data analysis will be done with the Statistical package for social sciences (SPSS) v 23 IBM Inc.17 Tests of normality will be carried out using the Shapiro-Wilks test for normality and Q-Q plots. Data will be reported as means and standard deviation for continuous data if they meet the assumption of normality. Data that is not normally distributed will be summarized as median value and interquartile range. Categorical data will be summarized as frequencies and proportions. The intention-to-treat principle will be followed. Chi square test (or Fisher's exact test as appropriate) will be used to compare categorical variables.
Comparison of continuous variables will be done with Students T test for normally distributed variables or Wilcoxon two-sample test for skewed variables. The effects of SC compared to UC on predefined and post-hoc pregnancy outcomes which include baby weight, prei-natal outcome, mode of delivery etc. as earlier defined will be expressed as relative risk (RR) and relative risk reduction and their 95% confidence intervals (CI).
CONCLUDING STATEMENT OF SIGNIFICANCE
Women who have gestational diabetes mellitus (GDM) constitute a significant proportion of subjects who eventually develop diabetes. Most pregnant women in the rural and semi-urban areas receive antenatal care at the primary health facilities and from traditional birth attendants with neither fundamental guidelines for screening and diagnosis of GDM, nor existing GDM screening practice. Thus, the significance of this trial is to provide data on the utility of universal GDM screening, and contribute additional epidemiological representative data for future planning and advocacy.

