Coastal Environmental DNA Sampling & Gravity Filtration Protocol
Alexandria B Boehm, Meghan M. Shea
Abstract
This is a protocol for collecting coastal environmental DNA (eDNA) samples and gravity filtering them on site, using a set-up with single-use enteral feeding pouches first described in Curd et al. 2019 (see Appendix S6) and further elaborated in Gold et al. 2021.
Steps
Pre-Sampling Preparation
Clean 1000 mL Nalgene bottles (as many as field blanks needed) with a 10% bleach rinse (leave for 10 minutes), then three rinses of DI water
Fill cleaned Nalgene bottles with 1000 mL of MilliQ water to use as field blanks
Sterilize luer lock to hose barb adapters, male-female luer lock caps, and silicone rubber cap
Place adapters and caps in a single layer in UV Crosslinker for 15 minutes
Store sterilized caps and adapters in sterile containers
Wipe down plastic storage bins with RNase Away, and organize needed field supplies (wiping individual items with RNase Away when possible as well).
- Sampling Infrastructure & Transport: clothes rack, bricks for stability, shade structure, beach cart
- Tools: tube cutter, flathead screwdriver, lab marker, temperature and salinity probe (e.g. Orion Model 1230 meter), prepared field blanks
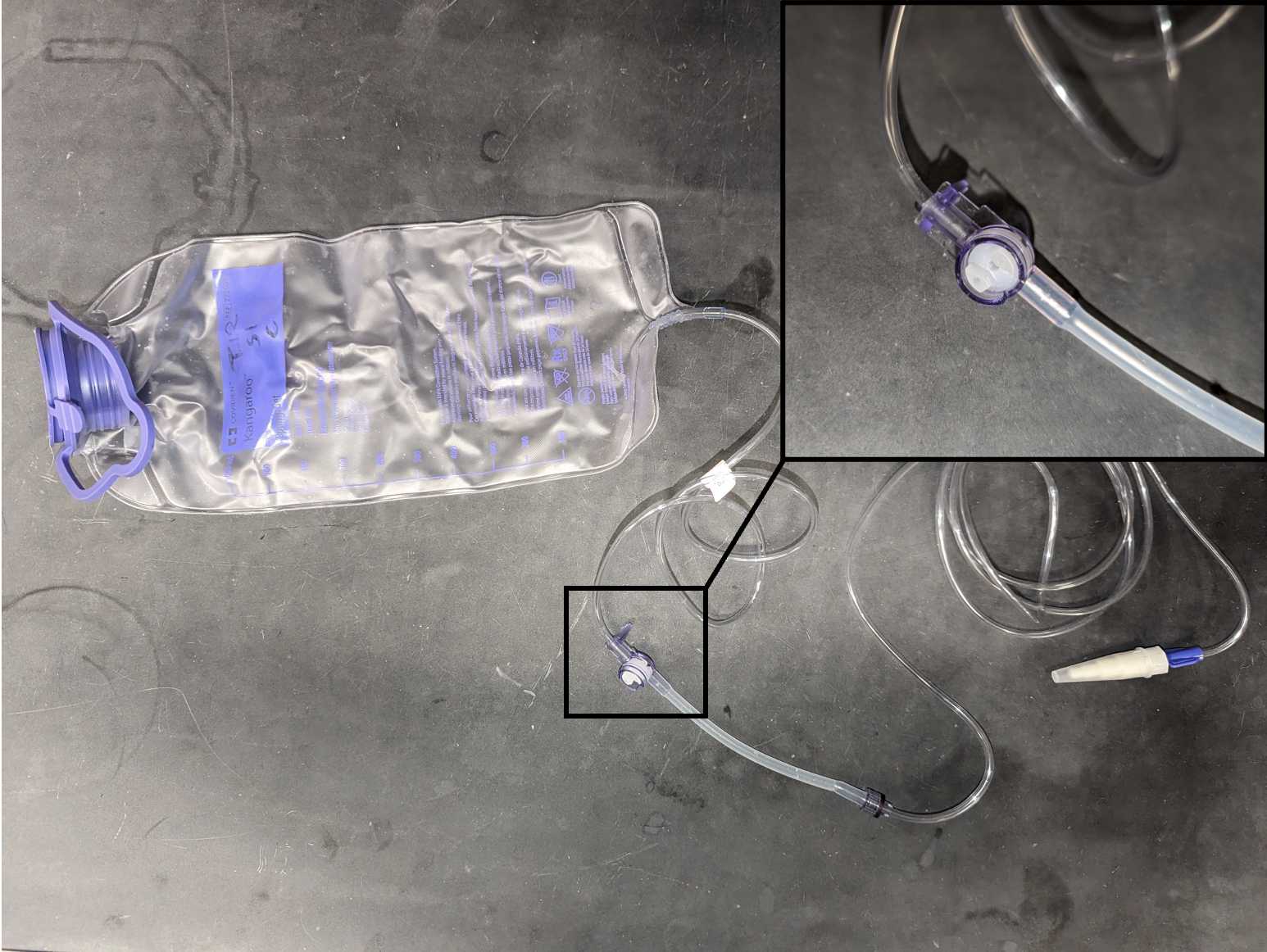
- Sampling Materials (1/sample) : zip ties, 1000 mL Covidien single-use enteral feeding pouches, sterile 0.22 μm Sterivex filters, male luer lock to hose barb adapters, dual male-female luer lock caps, round silicone rubber caps, sterile 3 mL luer lock syringes, small bungees, whirl-pak bags
- Preservation Materials: insulated cooler, ice packs
Sampling Set-Up
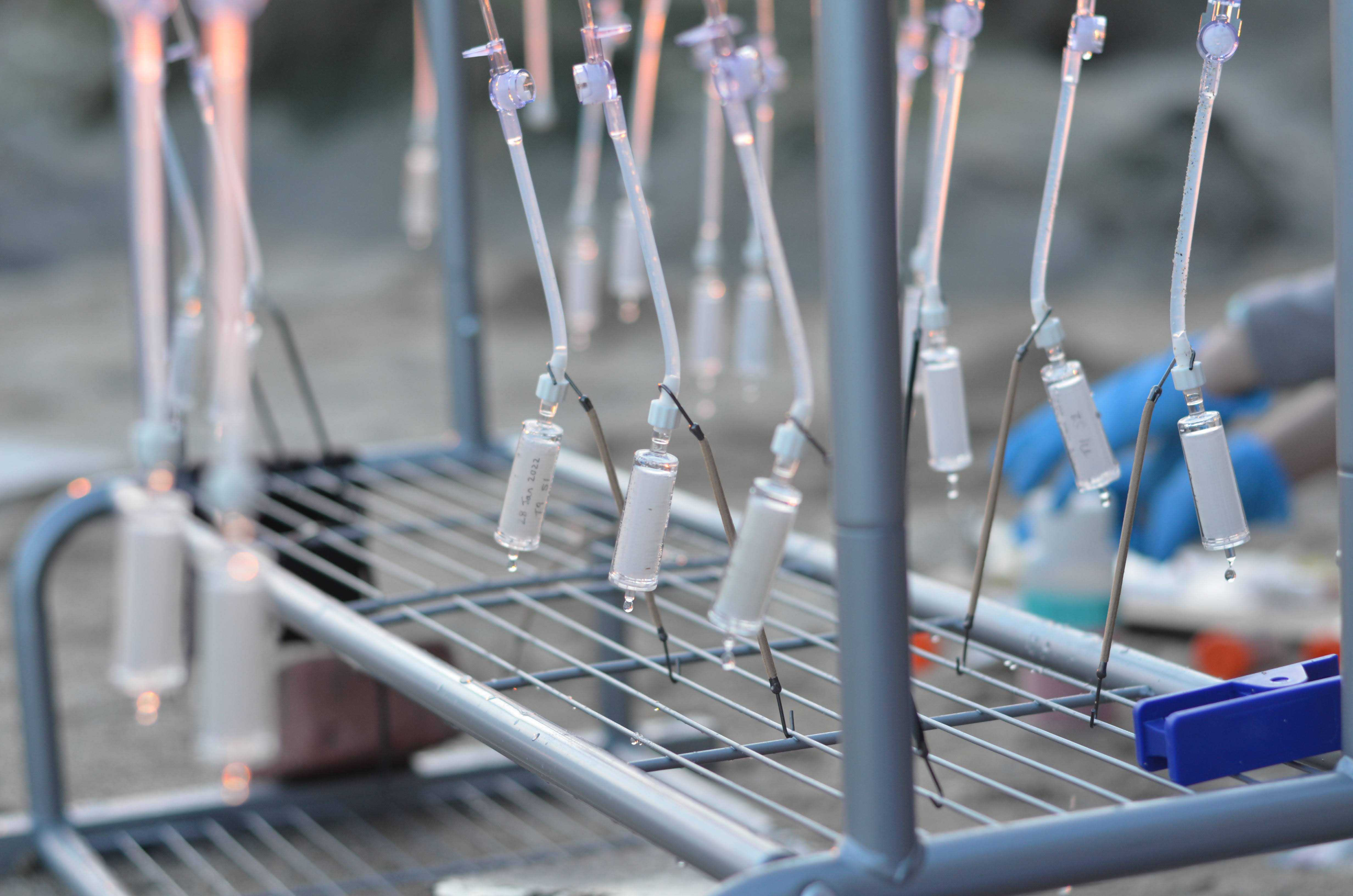
Put together clothes rack for hanging enteral feeding pouches for gravity filtration and weigh down with bricks. Set up shade structure to cover clothes rack.

Sampling
Put on fresh gloves
Label pouch with unique sample site/time identifier
Using temperature/salinity probe, record the temperature and salinity at the site the sample was collected
Secure the top of the pouch and return to clothes rack
Using a zip tie, secure the pouch to the top of the clothes rack

Put on fresh gloves, and grab one luer lock adapter and a Sterivex filter
Place the barb end of the luer lock adapter into the cut end of the tubing, remove the Sterivex filter, and lock the filter to the tubing
Label the filter with an ethanol-proof lab marker with a unique sample site/time identifier
Allow water to filter until all water has passed through (1-2 hours), or until drips slow to less than one/minute (clog); if the latter, record the amount of water that has passed through the filter
Remove the filter, and using a sterile 3 mL syringe, pass air through the filter until excess water has been removed
Cap the inlet end of the Sterivex filter with a male-female luer lock cap and the outlet end with a silicone rubber cap
Label a small Whirl-Pak bag with the unique sample site/time and place the filter inside
Place bag on ice until transporting to -15°C freezer at the conclusion of field sampling
Wrap-Up
If pouches need to be transported while still filtering:
Using a flathead screwdriver, twist the inside of the purple connector to stop the flow of water on any still-filtering bags
Using a flathead screwdriver, twist the inside of the purple connector to start the flow of water on any still-filtering bags. The cart can now be transported as pouches are still filtering.
Transport filters to -15°C freezer to await extraction