CZI Pediatric Nasopharyngeal Swab Processing for 10X scRNA-seq (Illustrated Protocol)
Jaclyn M Long, Erica M Langan, Ying Tang, Carly G.K. Ziegler, Vincent N. Miao, Andrew W. Navia, Joshua D. Bromley, Kenneth J. Wilson, Yilianys Pride, Mohammad Hasan, Taylor Christian, Hannah Laird, Anna Owings, Meredith Sloan, Haley B. Williams, Tanya O. Robinson, George E. Abraham III, Michal Senitko, Sarah C. Glover, Bruce Horwitz, Alex K. Shalek, Jose Ordovas-Montanes
Disclaimer
DISCLAIMER – FOR INFORMATIONAL PURPOSES ONLY; USE AT YOUR OWN RISK
The protocol content here is for informational purposes only and does not constitute legal, medical, clinical, or safety advice, or otherwise; content added to protocols.io is not peer reviewed and may not have undergone a formal approval of any kind. Information presented in this protocol should not substitute for independent professional judgment, advice, diagnosis, or treatment. Any action you take or refrain from taking using or relying upon the information presented here is strictly at your own risk. You agree that neither the Company nor any of the authors, contributors, administrators, or anyone else associated with protocols.io, can be held responsible for your use of the information contained in or linked to this protocol or any of our Sites/Apps and Services.
Abstract
Before start
Attachments
Steps
Before You Start
Record sample characteristics in Table 1
| A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|
Final Set-Up
Timing
Fill out timing chart at indicated steps of protocol
| A | B |
|---|---|
| Start time: | |
| Time after preparation of single cell suspension: | |
| Time after counting: | |
| Time 10X controller run started: | |
| Time 2nd 10X controller run started (if applicable): | |
| Time lysis buffer added for bulk lysates: |
Tube A (the cryovial)
Rapidly (within 1-2 minutes) thaw cryovial ( Tube A ) in thermal block set to 37°C.
Remove swab from Tube A using clean forceps.
Dip swab in Tube B (15 mL conical) once. Swirl briefly (approximately 3 seconds) to rinse swab.
Move swab from Tube B (15 mL conical) to Tube C . Trim swab handle using scissors if necessary so that it can fit inside a 1.5mL tube (see video). Proceed directly to step 27 for Tube C . In parallel, continue processing Tube B .
Transfer liquid in Tube A to Tube B (15 mL conical).
Using 1mL from Tube B (15 mL conical), wash Tube A .
Collect washing from Tube A in Tube B (15 mL conical). Continue below with Tube B
Discard Tube A .
Tube B
Centrifuge Tube B (15 mL): 400x g,4°C.
Remove supernatant with serological pipette or P1000. Do not discard supernatant.
Transfer supernatant to Tube F .
Resuspend pellet in 1mL.
Transfer suspended cells from Tube B (15 mL) to Tube B (1.5 mL). Discard empty 15 mL conical.
Place Tube B (1.5 mL) on thermomixer (37ºC, 300 rpm).
Incubate for 0h 15m 0s. In parallel, continue processing Tube C.
Centrifuge Tube B (1.5 mL): 400x g,4°C.
Remove supernatant with P1000 pipette. Discard supernatant.
Resuspend pellet in 1mL.
Place Tube B (1.5 mL) on thermomixer (37ºC, 300 rpm).
Incubate for 0h 30m 0s.
Tube C
Place Tube C on thermomixer (37ºC, 300 rpm).
Incubate for 0h 15m 0s.
Place swab in Tube D . Proceed directly to step 36 for Tube D .
Centrifuge remaining liquid at 400x g,4°C.
Remove supernatant with P1000 pipette. Do not discard supernatant.
Place supernatant in Tube F. Keep Tube F on ice until you proceed to Step 71.
Resuspend pellet in 1mL.
Place Tube C on thermomixer (37ºC, 300 rpm).
Incubate for 0h 30m 0s.
Tube D
Place Tube D on thermomixer (37ºC, 300 rpm).
Incubate for 0h 30m 0s.
After Tube B, C, and D's 30 minute Incubations
After Tube B , Tube C , and Tube D have each finished their 30 minute incubations:
Place 70 µm filter in 50 mL conical.
Wet filter with 3mL.
Pipette contents of Tube B , Tube C , and Tube D onto filter with P1000 pipette
Step 41 - adding Tubes B,C,D contents to filter.mp4
Use 1mL to wash each Tube B , Tube C , and Tube D .
Manually agitate the swab in Tube D in the quenching media with forceps to ensure full rinse.
Add quenching buffer from washes to filter. Discard Tubes B , C , and D .
Wash filter with additional 2mL.
Discard filter, cap 50 mL conical.
Remove supernatant with serological pipette. Leave ~500 µL in the bottom of the tube.

Add 500 µL RPMI + 10% FBS to the tube to resuspend cells.
Transfer resuspended cells (~ 1 mL) from 50 mL conical to Tube E (1.5 mL tube).
Wash 50 mL conical with 500µL.
Transfer washing from 50 mL conical to Tube E .
Centrifuge Tube E 400x g,4°C.
Remove supernatant with P1000 pipette.
Centrifuge Tube E 400x g,4°C
Resuspend pellet in 200 µL RPMI + 10% FBS
Count cells from Tube E
In a new empty 1.5 mL tube, add 10µL.
Add 10µL to 1.5 mL tube containing trypan blue.
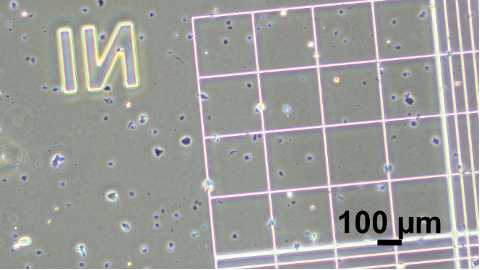
Pipette to mix cells in trypan blue, transfer 10µL to hemocytometer port.
Count viable cells across 8 quadrants. One quadrant is the corner of a hemocytometer grid, consisting of 16 smaller squares.
Record total cell number and calculate cell concentration using the "Cell Counts & 10X Volumes" sheet of the Calculation Tables template.
Prepare cells for 10X
Prepare 1.5 mL tube for each pool
For each sample, add "Volume Necessary for Desired Number of Cells" from "Cell Counts & 10X Volumes" sheet of the Calculation Tables template to correct pool.
Pipette cells to mix
If volume of pool is > 100 µL, centrifuge cells 400x g,4°C , then resuspend in 500µL and proceed to next step.
If volume of pool is < 100 µL, add 500µL directly to pool and proceed to next step.
Centrifuge cells 400x g,4°C
Resuspend cells in 43.3µL (Total volume of cell suspension + water on page 27 of 10X Protocol).
Add 43.3µL directly to 31.9µL (Step 1.2b of 10X Protocol)
Proceed with instructions in 10X Protocol to load the chip and run the controller (through Step 1.3)
Tube F Processing (Viral Lysates)
Remove a 200uL aliquot from Tube F and transfer to a new, empty 1.5mL tube. Store at -80C for antibody studies.
1mL to Tube F. Distribute contents of Tube F into 3 cryovials per sample
Snap freeze on dry ice
Store at -80°C
Lysates for Bulk RNA-seq
For each lysate, add "Volume Necessary for Each Lysate" from "Bulk RNA Lysate Volumes" sheet of the Calculation Tables template to one well of a 96-well PCR plate according to the Lysate Storage Plate map.
Seal plates and centrifuge 400x g,4°C
Aspirate media
Resuspend cells in 50µL . Mix with pipette. Bubbles are okay here.
Seal plate with foil seal and spin down briefly.
Place plate on dry ice for 0h 15m 0s -0h 20m 0s to snap-freeze lysate.
Store at -80°C until ready to use.