Bioinformatics Analysis of Estrogen-Responsive Genes
Adam E. Handel
Abstract
Estrogen is a steroid hormone that plays critical roles in a myriad of intracellular pathways. The expression of many genes is regulated through the steroid hormone receptors ESR1 and ESR2 . These bind to DNA and modulate the expression of target genes. Identification of estrogen target genes is greatly facilitated by the use of transcriptomic methods, such as RNA-seq and expression microarrays, and chromatin immunoprecipitation with massively parallel sequencing (ChIP-seq). Combining transcriptomic and ChIP-seq data enables a distinction to be drawn between direct and indirect estrogen target genes. This chapter discusses some methods of identifying estrogen target genes that do not require any expertise in programming languages or complex bioinformatics.
Attachments
Steps
3.1 The Genomic HyperBrowser
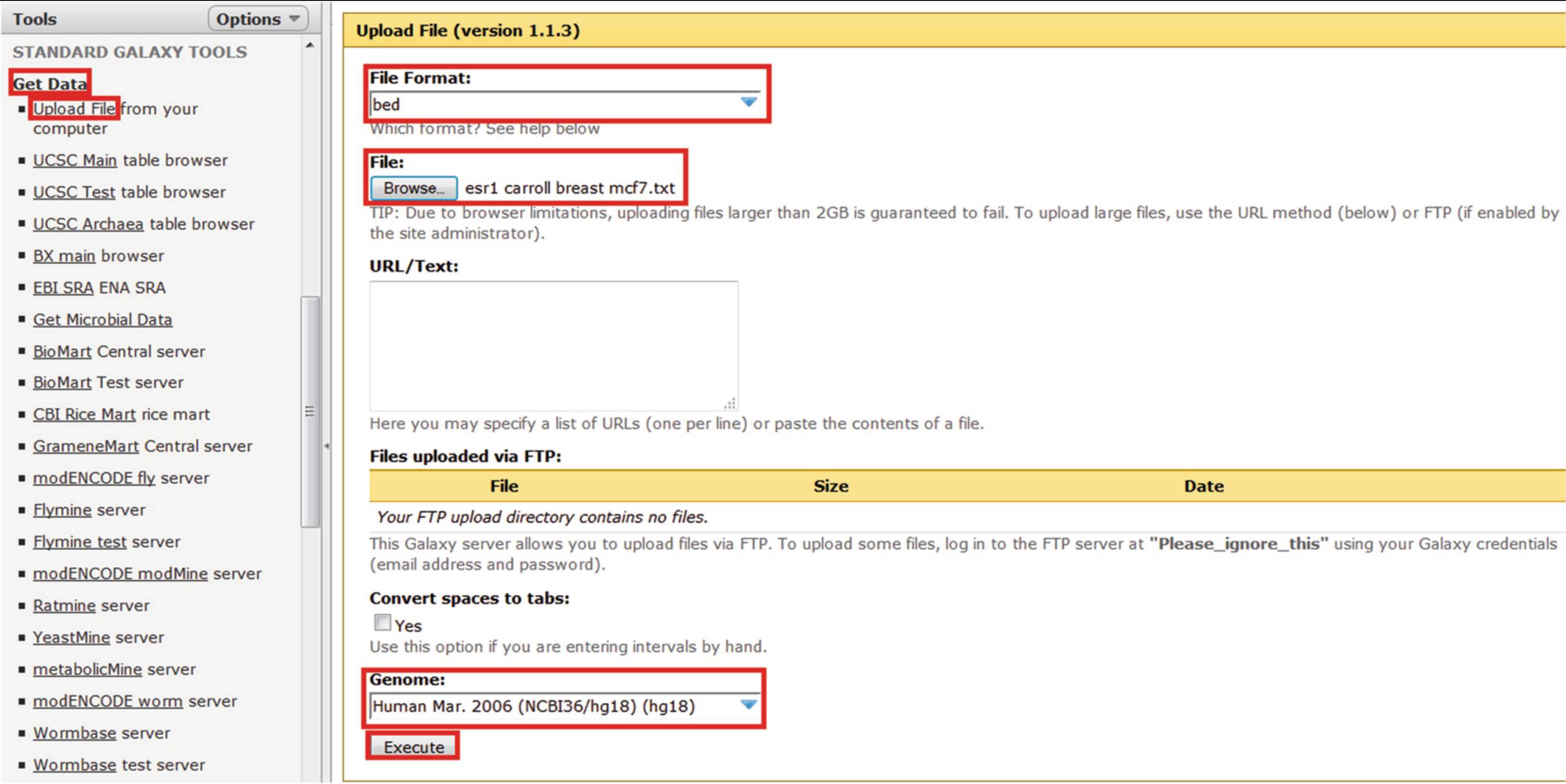
Register for the Genomic HyperBrowser (https://hyperbrowser.uio.no/hb/).
Prepare ChIP-seq and transciptomic datasets for upload. ChIP-seq files should be a set of tab-delimited genomic coordinates corresponding to each peak in the format:
Chromosome Start Stop
Transcriptomic files should be a list of differentially expressed genes (DEGs) as ENSEMBL IDs ( see Note 1 for methods for converting between different forms of gene ID). The datasets used for this demonstration are the ESR1 binding site data (actually ChIP-chip data) from Hurtado and colleagues and transcriptomic data from Hah and colleagues (thresholded at q < 0.05) [6, 19].

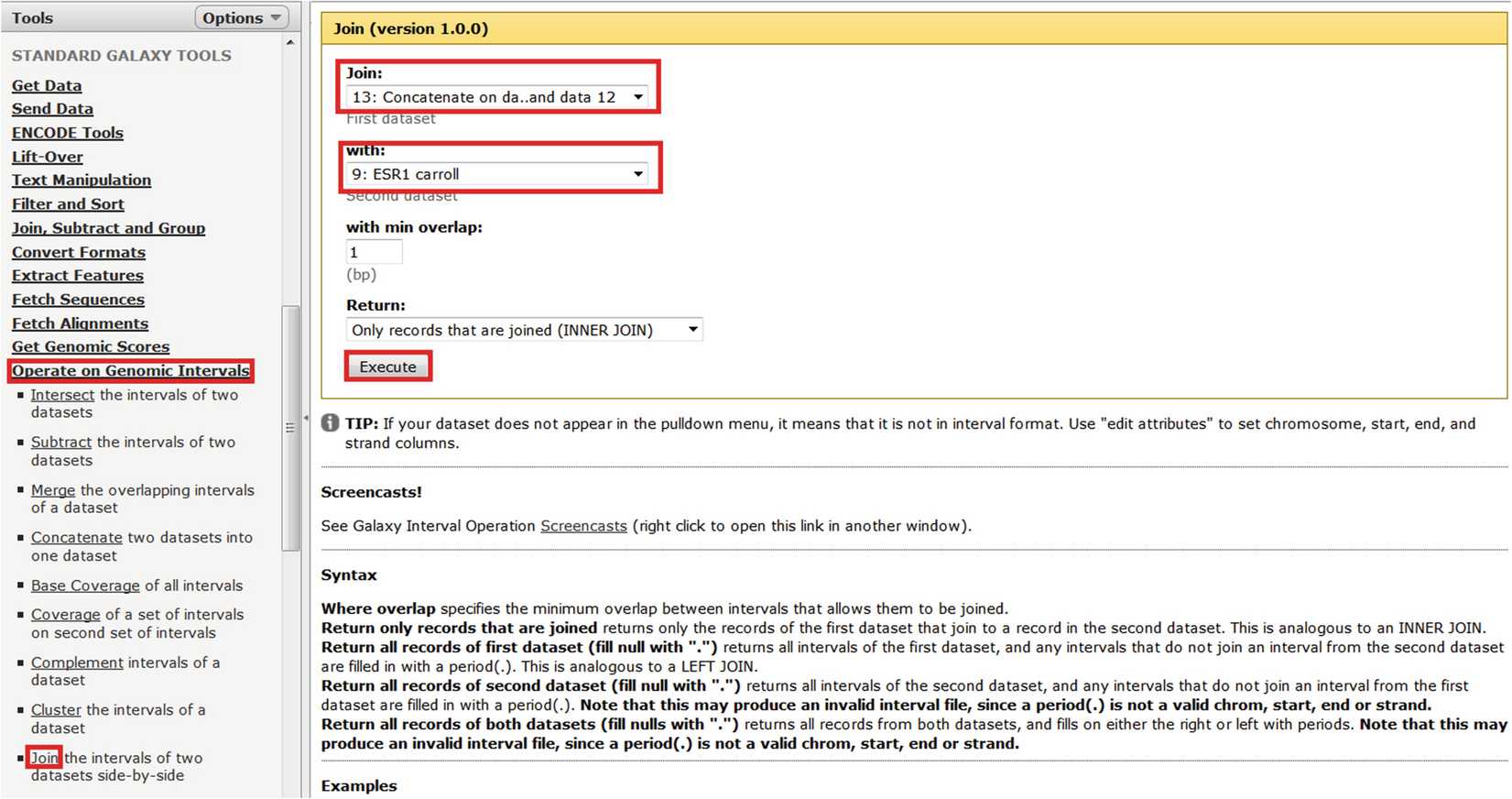
Firstly upload the ChIP-seq data as shown in Fig. 1.
Next use “Generate Tracks > Generate segment track from gene IDs” to obtain genomic intervals from the ENSEMBL gene IDs of DEGs. These should be uploaded into the tool as a series of comma-separated values as shown by the demo data. If necessary genomic intervals can be lifted from one genome build to another by the “Lift-Over > Convert genomic coordinates” tool.
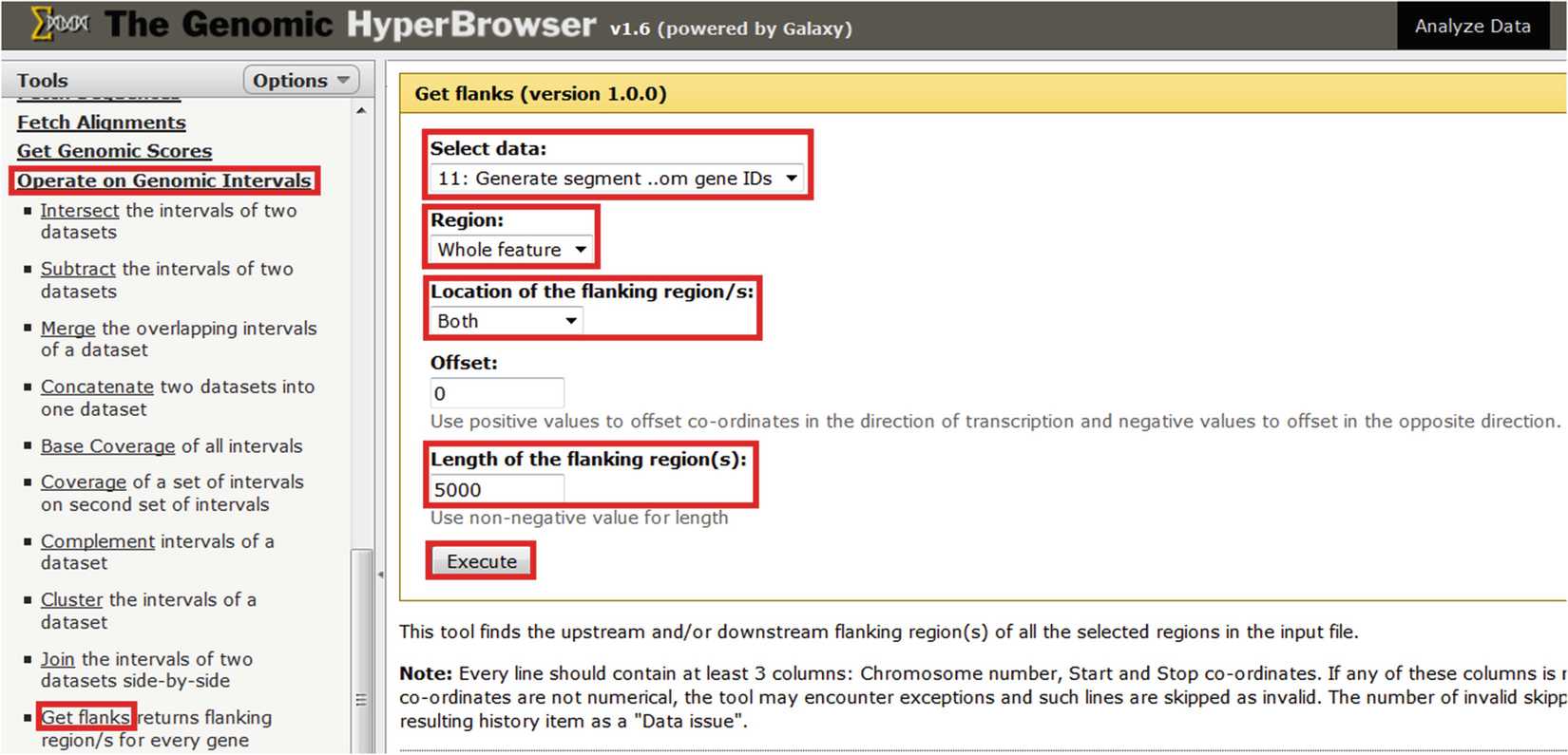
Use “Operate on Genomic Intervals > Get flanks” to extend gene regions by a pre-specified number of bases in each direction (Fig. 2). A suitable distance might be 5 kb, which is analogous to the upstream region extension in the gene ontology tool GREAT [20].
The original intervals and flanking regions should then be concatenated into a single track using “Operate on Genomic Intervals > Concatenate” and then merged with “Operate on Genomic Intervals > Merge.”
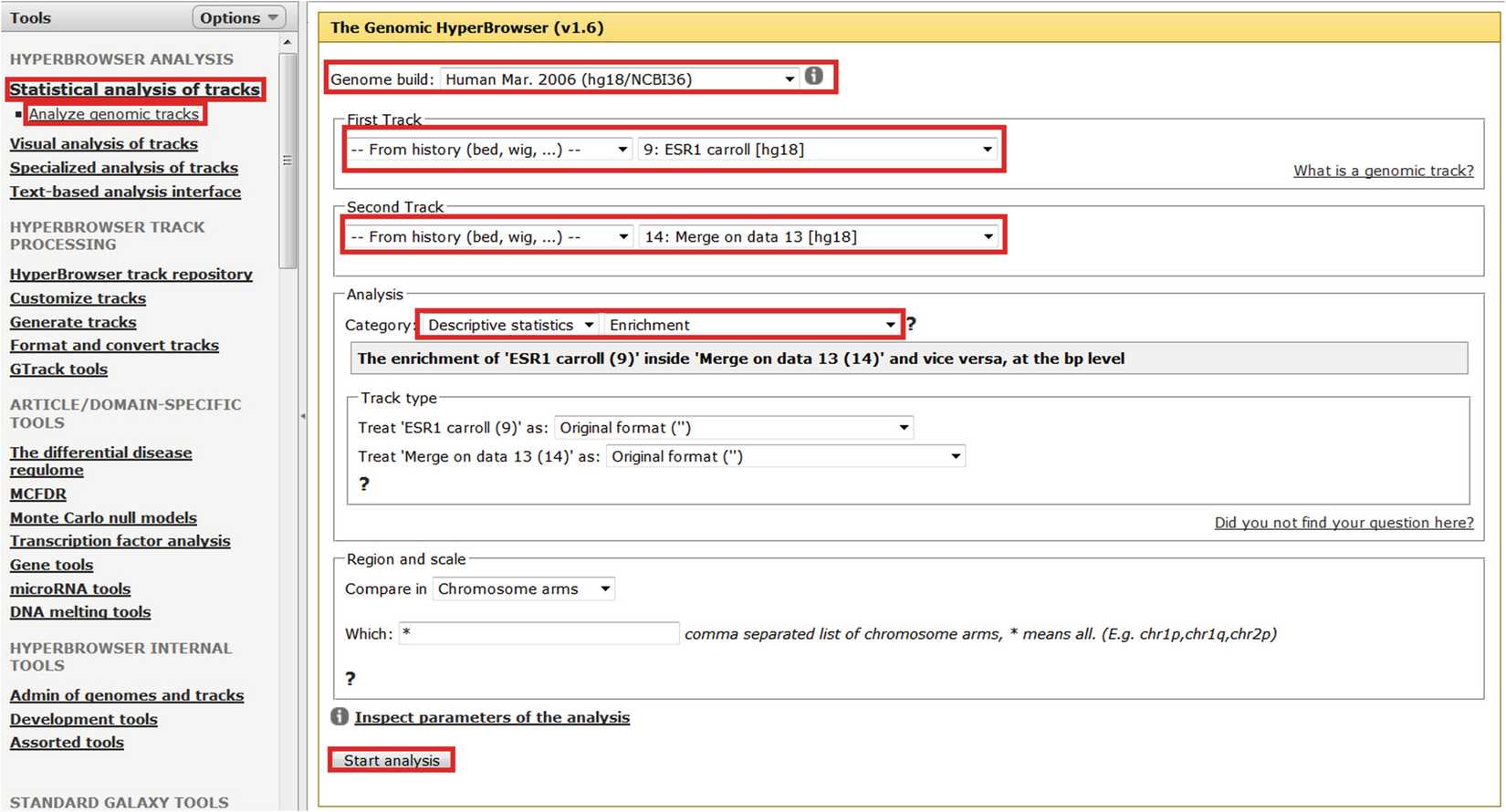
An important sanity check is to ensure that estrogen receptor binding is enriched near estrogen DEGs. The Genomic HyperBrowser allows one to calculate the enrichment of estrogen receptor-binding sites with the intervals generated above (i.e., within 5 kb of estrogen DEGs). Figure 3 illustrates this process. It is possible to use the Genomic HyperBrowser to calculate an empirical p-value for this overlap using the same tab as for enrichment analysis but selecting “Category: Hypothesis testing,” “Overlap?,” a suitable null model (e.g., “Preserve segments (T2), segment lengths and inter-segment gaps (T1); randomize positions (T1) (MC)”) and the number of permutations (e.g., for publication quality p-values ~10,000 permutations would be recommended). The region and scale tab is also important as this determines in which areas of the genome randomized tracks can fall. Leaving it at its default value (all chromosome arms) is adequate for the current sanity check. There is significant overlap between ESR1-binding sites and estrogen DEGs (2.14-fold, p < 10−4), which suggests that there are likely to be plausible direct estrogen targets amongst the transcriptomic dataset. Note that analyses are only conducted on bed files, and so if the track of interest is not offered by the Genomic HyperBrowser as a potential track for analysis then edit that track to ensure that the track type is “bed.”
The resultant output can be pasted into a spreadsheet program and filtered to obtain unique gene IDs and their respective ESR1-binding sites.
3.2 BETA
Register for Galaxy/Cistrome (http://cistrome.org/ap/). This tool integrates transcription factor-binding sites with the degree of differential gene expression to predict high-confidence direct targets.
Prepare ChIP-seq and transcriptomic data for upload. Again, the ChIP-seq data should be a tab-delimited file in the format:
Chromosome Start Stop
RNA-seq data can either be directly uploaded as Cuffdiff or LIMMA output [21, 22] or formatted as a tab-delimited file with columns corresponding to gene ID, direction of change (e.g., T-score ) and significance (e.g., FDR). Ensure that all data are present as text (gene ID) or numerical data. Some spreadsheet programs can format data in ways that will cause BETA to crash (e.g., substituting dates for numerical values).
Start the BETA tool running after selecting the appropriate parameters (Fig. 5). The BETA tool is available through “Integrative Analysis > BETA-plus: Binding and Expression Target prediction and motif analysis.” For an initial analysis, it is recommended to leave as many settings at their default values as possible. Subsequently these can be altered to test how robust the results are to changes in, for example, the distance threshold of ESR1-binding sites to DEGs.

The output files then produce direct target predictions. These are described below:
- BETA functional prediction on ESR1 ChIP-chip: A graph showing the relationship between functional rank and the number of direct targets and an associated p-value for upor downregulated genes.
- BETA direct targets prediction on up regulated genes: A table of up-regulated gene targets detailing the rank product score (derived from the significance score provided in the transcriptomic dataset).
- BETA direct targets prediction on down regulated genes: A table of downregulated gene targets detailing the rank product score (derived from the significance score provided in the transcriptomic dataset).
- Uptarget associated peaks: A list of peaks with the associated up-regulated gene target, the distance to the target gene, and a functional score.
- Downtarget associated peaks: A list of peaks with the associated downregulated gene target, the distance to the target gene, and a functional score.
- Motif analysis on target regions: An html output file detailing top motifs detected for multiple comparisons along with associated statistical scores.
- A series of detailed motif analysis outputs: The statistical data for the above file.
- Log of BETA plus: This details the input parameters and any errors encountered during the course of the analysis.