Automated combinatorial media preparation
Tijana Radivojevic, Matthew Incha, vblayroger, Apostolos Zournas, Stephen Tan, Hector Garcia Martin
Abstract
Media optimization is a critical, and often overlooked, process which is essential to obtain the titers, rates and yields needed for commercial viability. Here, we present a molecule- and host-agnostic active learning process for media optimization that is enabled by a fast and highly repeatable semi-automated pipeline. Its application yielded 148% and 170% increases in titer, and 300% increase in process yield in three different campaigns for flaviolin production in Pseudomonas putida KT2440. Explainable Artificial Intelligence techniques pinpointed that, surprisingly, common salt (NaCl) is the most important component influencing production. The optimal salt concentration is very high, comparable to seawater and close to the limits that P. putida can tolerate. The availability of fast Design-Build-Test-Learn (DBTL) cycles allowed us to show that performance improvements for active learning are rarely monotonous. This work illustrates how machine learning and automation can change the paradigm of current synthetic biology research to make it more effective and informative, and suggests a cost-effective and underexploited strategy to facilitate the high titers, rates and yields essential for commercial viability.
Here we provide the protocol for automated media optimization on a flaviolin producing Pseudomonas putida KT2440 strain.
Before start
Make sure you booked the equipment in the calendar:
- 978-PRO-BIOLECTOR (4148) EQ and
- 978-4-BIOMEK-NX-S8_ SPECTRAMAX (4148) EQ (1) or
- 978-4-BIOMEK-NXP (4148) EQ (1)
Make sure you received proper training before operating on the Biomeks and Biolector.
Have an overnight of your culture ready.
Steps
Required equipment/labware
Liquid handler:
Equipment
| Value | Label |
|---|---|
| Beckman Coulter | BRAND |
| Biomek NXp | SKU |
Pipette tips needed (available at Robotics lab):
- tips f200 (light green box)
- tips f20 (light blue box)
Fermentation platforms available:
- [Biolector]
- Biolector Pro or
- Multitron
Additional components:
-
- Kanamycin
To be prepared fresh for every cycle:
-
- Overnight culture of P. putida PP_5404::attB::pIS100. (The base strain for this assay was simply KT2440 modified for PhiC31 integrations.)
Additional labware:
- 5mL, 10mL pipettes
- 3x 50mL tubes for FeSO4
- filter, syringe for FeSO4 sterilization
- 2x 2mL tubes for culture
Additional equipment:
- vortex
- centrifuge
- flame
Calculate stock concentrations
Input:
- total volume of the culture (we use
1500µLas total volume) - standard media recipe (e.g. standard_recipe_concentrations.csv)
Run the notebook A_Find_Stock_Concentrations.ipynb.
Prepare stock solutions
Use the list of components concentrations created in step 5 and prepare stock solutions. Ideally, you should prepare enough volume for all DBTL cycles.
Create stock plates definitions
Run the notebook B_Create_Stock_Plates.ipynb. Prepare the stock plates following the instructions resulting from the notebook by aliquoting the components into the appropriate wells of the source plate. A stock plate with culture and FeSO4 should be prepared fresh for every cycle. The other two, with high and low level concentrations, can be used and refilled if needed for consecutive cycles.
Example output files:
- source plate instructions 24-well_stock_plate_high.csv 24-well_stock_plate_low.csv 24-well_stock_plate_fresh.csv
An example of how to prepare a stock of Kanamycin:
- Take 1000x concentration from the freezer
- If we need e.g.
1mLof the 300x concentration in the stock plate, we need a 3.33 dilution, so300µLof Kan and 1000-300=700µLof H2O
Choose target media composition
E.g. by using ART. For the initial cycle you may run the notebook C_Initial_Media_Designs.ipynb. Once there is a training data set you may run the notebook C_ART_Media_Designs.ipybn.
Example output file: target_concentrations.csv
Create files for biomek transfers and source plate instructions
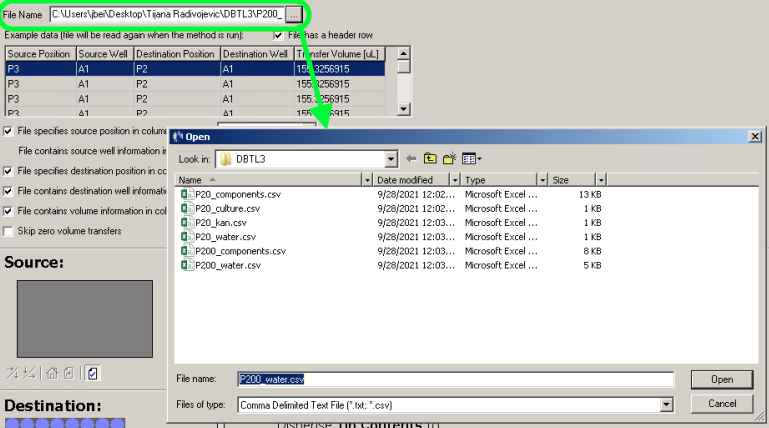
Run the notebook D_Create_Transfers.ipynb. It will generate a file with volumes for all components for all wells, files for biomek that are used to define transfers of those volumes, and stock plate definitions with additional columns with required volumes for each stock component, for this particular run.
Example output files:
- destination volumes dest_volumes.csv
- source plate instructions 24-well_stock_plate_high.csv
- biomek files P20_components.csv P20_culture.csv P20_kan.csv P20_water.csv P200_components.csv P200_water.csv
This notebook will also provide a number of tip boxes needed to perform the transfers. Most likely, the number of boxes will be larger that the number of slots on the Biomek deck, so the method will need to pause, which gives you time to refill the deck with tips.
Prepare the source plates
Make sure the stock plates with low and high concentration levels, prepared at step 7, have at least volume levels as defined in step 9.
FeSO4 :
-
To prepare
20mLof60millimolar (mM)stock, find molar mass of the iron-sulfate available and use a calculator to find mass in mg -
Typical stock is FeSO4*7H2O aka the heptahydrated salt. 333.612mg heptahydrate / 20mL sterile H2O to make 60mM stock
-
Measure and add FeSO4*XH2O into a 50 mL falcon tube, add
20mLof H2O, vortex -
The solution may be cloudy before the filtration. This is because of some oxidized FeSO4 (new insoluble Fe(III) species) in the solution. Do not be alarmed. This is a small proportion of the stock iron sulfate that was measured.
-
Use a sterile 0.2uM luer-lock syringe filter (or SteriFlip if available) to filter sterilize the 60mM solution into a new 50mL conical tube
-
Find volume of this solution needed to create 10 mL of solution with target concentration of
0.3millimolar (mM)using the formula:9.e. 10mL * 0.3mM / 60mM = 0.05 mL of FeSO4 -
Add
9.95mLof H2O to another (falcon) tube and0.05mLof FeSO4, vortex, filter sterilize
Culture:
- Take 2mL of overnight culture and place into the 2mL tube (2x)
- Centrifuge the 2 2mL tubes of culture at
10000rcf - Decant the supernatant
- Re-suspend the overnight culture with an equal volume MOPS minimal medium without carbon
- Place the resuspended culture in the appropriate well of the source plate
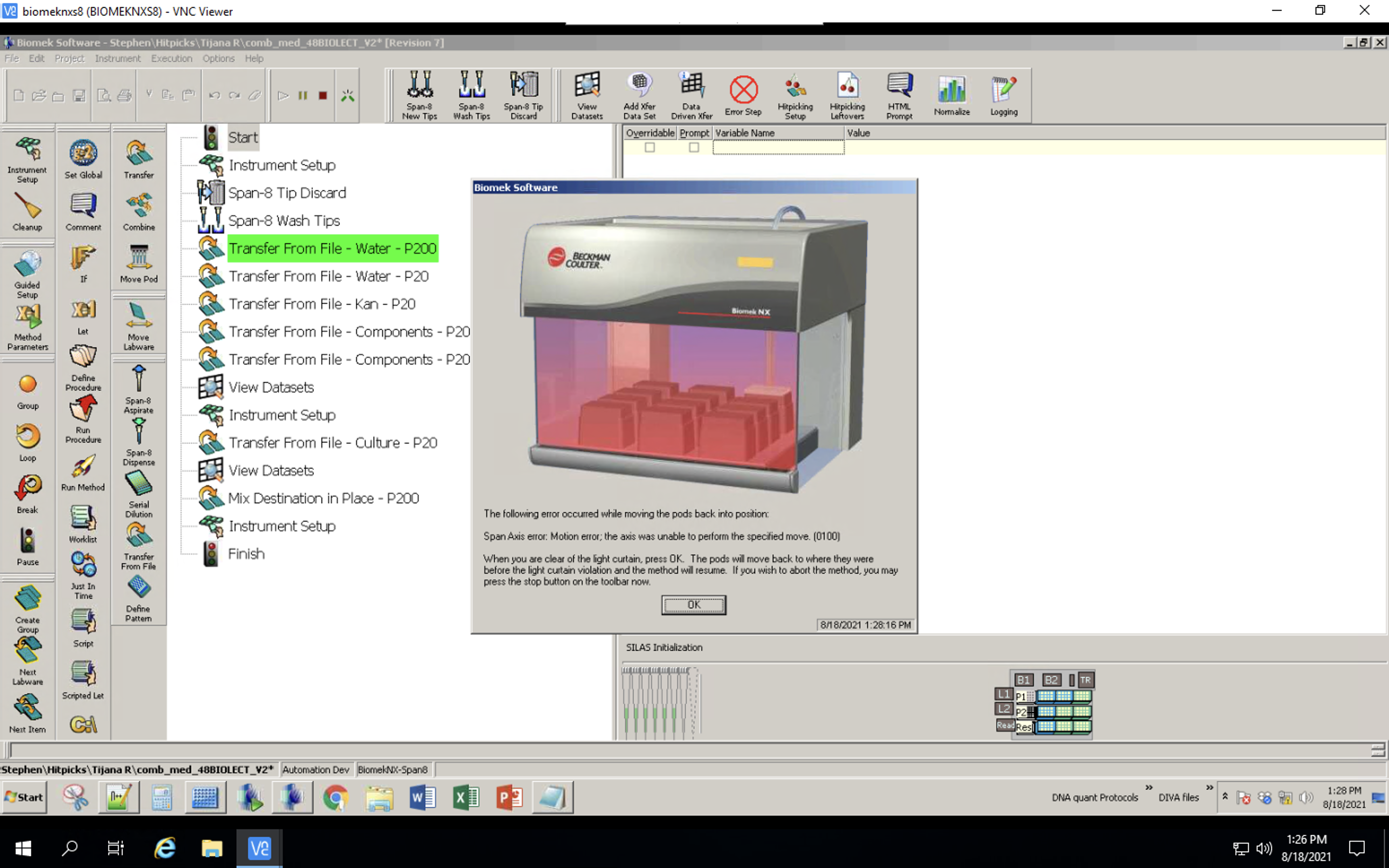
Biomek setup
Set up the deck as specified by the Instrument Setup pop-up. Check if the number of tip boxes corresponds to what was calculated in step 9, notebook D_Create_Transfers.ipynb.
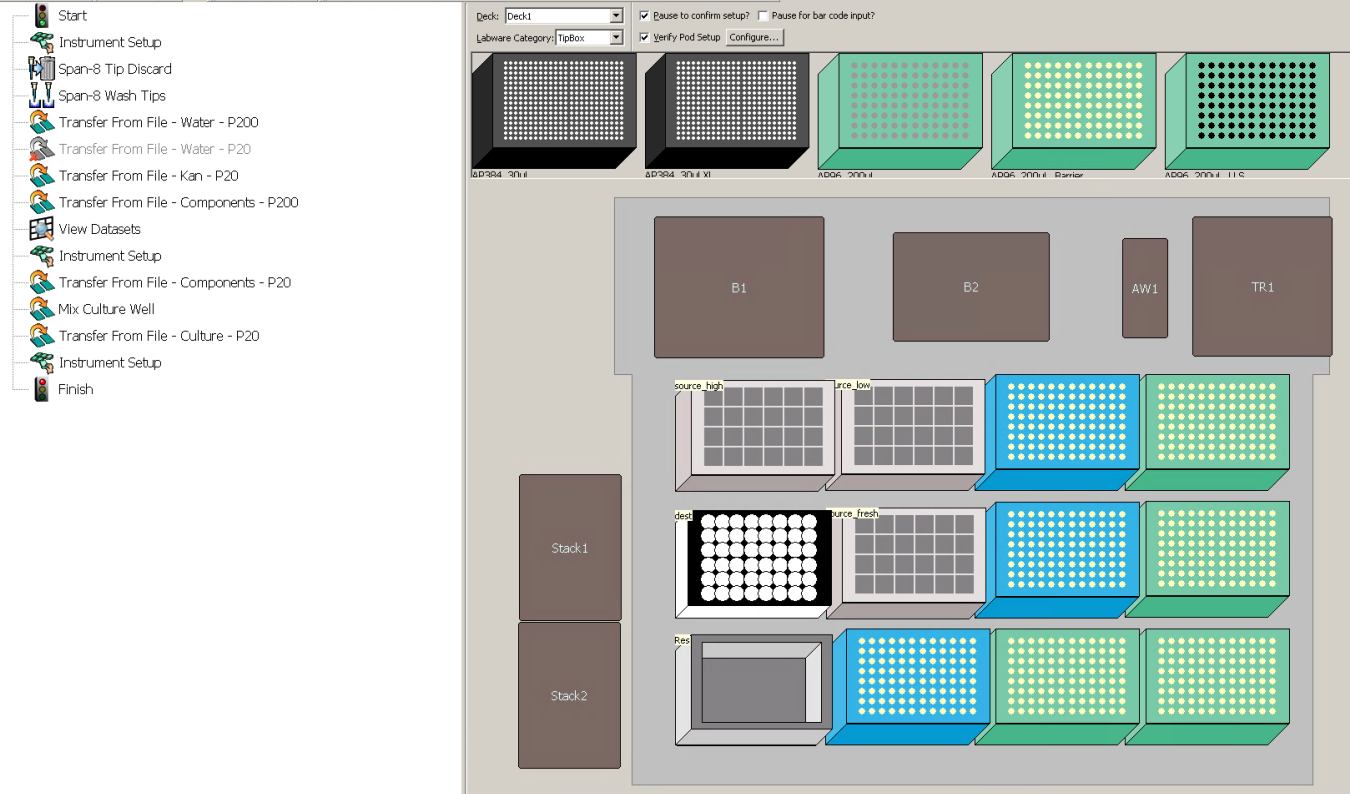
Check that the deck layout after the pause (second "Instrument Setup" step) corresponds to the number of tip boxes calculated in Step 9, notebook D_Create_Transfers.ipynb.
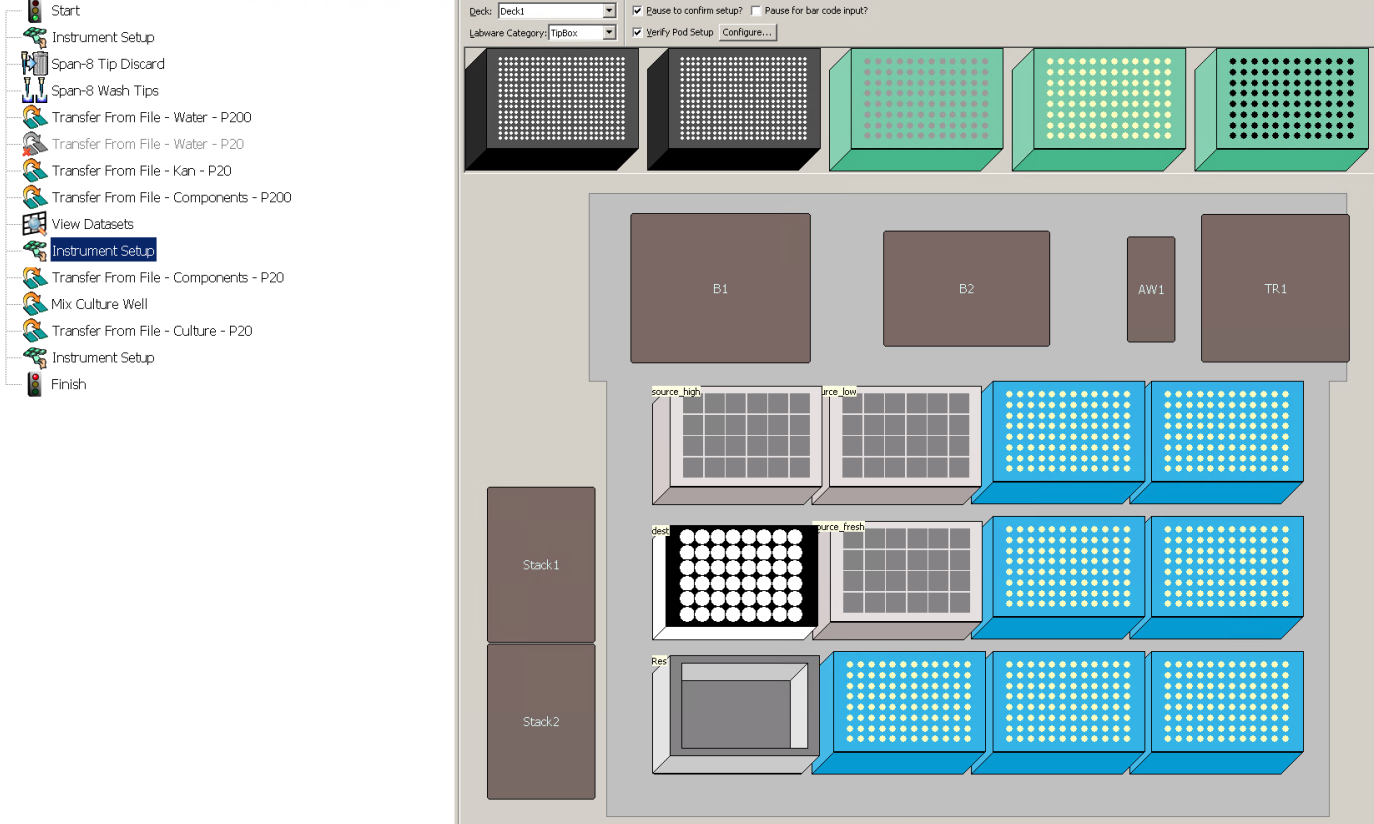
Biomek simulation
Biomek run
Biolector/Multitron run
On the Biolector, open the protocol labelled "matthew_flower".
Adjust the calibration "lot number" to that which corresponds to the sticker on the M2P biolector flower plate.
If the lot number is unavailable, import it and load it into the matthew_flower method.
(you should have been trained on this. if you can't remember how, email the current biolector superuser)
Place the plate inside the machine and start the method.
Temperature 30°C
Humidity 80%
Length of the run 48h 0m 0s
Shake Speed (800 rpm)
Filters:
- ID401 (620nm for biomass measurement)
- ID402 (pH measurement)
- ID403 (DO measurement)
On Multitron:
Temperature 30°C
Shake speed 700RPM
Place plate in Infors Multitron
Absorbance assay
Follow a protocol for absorbance assay from an m2p plate.
Upload data into EDD
Run the notebook E_Create_EDD_Study_Files.ipynb.
Once you create files for EDD import, follow a protocol for EDD study creation and a protocol for importing data into EDD.