Application of PHYTO-PAM-II (Compact Version) on Aureococcus anophagefferens cultures for photosynthetic efficiency and quantum yield of PSII
Alex Truchon, Steven W Wilhelm, Emily E. Chase, Gwen Stark
Aureoccocus
photosynthetic efficiency
quantum yield
Fv/Fm
algae
brown tide
HAB
chlorophyll fluorescence
Abstract
A protocol used to acquire photosynthetic efficiency (Fv/Fm) and quantum yield of photosystem II (Y(II)) of Aureococcus anophagefferens cultures. A maximal Fm (and minimal Fo) is acquired by dark adaption of the cultures beforehand.
Before start
Familiarise yourself with the PHYTO-PAM-II equipment, manufacturer's provided manual, and the basics of chlorophyll fluorescence parameters for full use of data collected and accuracy of results.
Steps
SAMPLE PREPARATION
Prepare 3 mL of each culture of Aureococcus anophagefferens to be measured in appropriately labelled, separate 15 mL Falcon tubes. Additionally, prepare at least one sample to carry out the auto-gain function if you do not know the appropriate gain (see Step 3.1 ).
For acquiring Fo and Fm, dark adapt all samples for 20–30 minutes. Both quantum yield (Y(II)) and photosynthetic efficiency (Fv/Fm) can be collected without dark adaption, but will be deduced when the photosystem II has not been completely cleared. Dark adaption is completed to obtain the maximal possible value of fluorescence (Fm) upon light application.
EQUIPMENT PREPARATION
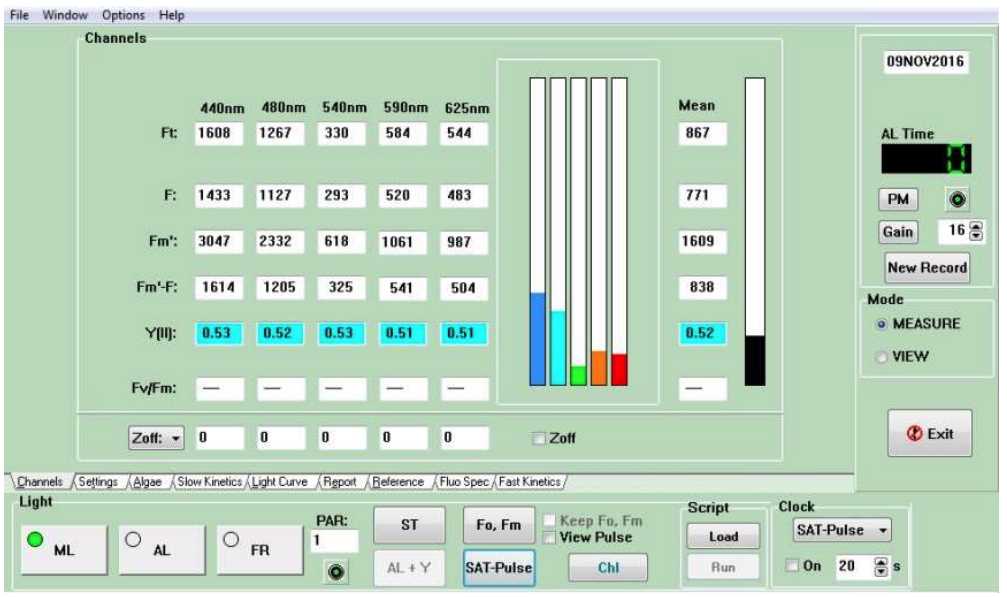
Start the PhytoPAM-II Compact Version up by toggling the power, and plugging in the charger. Connect the equipment to a laptop or computer by USB. Select the PhytoWin_3 program from the computer, select the appropriate measuring head you are using (Compact Version = "Phyto Compact Unit"). See Figure 1 for the software interface. In order to take measurements ensure that the program is in "MEASURE" mode. "VIEW" mode is exclusively for reviewing record files ( i.e., data collected). Ensure the "ML" (measuring light) light is selected (signalled by a green light), and that "AL" (actinic light) and "FR" (far red) are not selected. Wait for the light directly under the PAR input to be green before proceeding with any measurements (if the equipment is not ready the light will be red). If you have not deduced a gain to use during measurements then proceed to step 3.1, otherwise proceed to Step 4 .
Transfer 3 mL of culture sample (dark adapted or not) to the measuring cuvette, wipe down the sides of the cuvette with a KimWipe, place the cuvette into the measuring head, replace the lid, and click "Gain" on the measurement green (right side). This will provide a gain automatically that will work well with your sample.
MEASUREMENT ACQUISITION
Experimental measurements can now be collected. Set the gain to the appropriate level.
Add 3 mL of the culture to be measured into the cuvette, wipe down the sides with a KimWipe, place into the measuring head and cover with the cap. Wait for the indicator lamp to turn green (from red). Press the "Fo, Fm" button.
Measurements have now been recorded in the "Channels" and "Algae" tab. Check relevant wavelengths and/or "Algae" settings for comparing samples/treatments. Once completed click "New Record" before insertion of the next sample. Ethanol (70%) should be used to clean the cuvette between different treatments.
Acquire data for all samples, record, and shut down the program, toggle the machine off, and unplug the charger and USB connection.

