A systematic review protocol for Biometrics in healthcare: strategies for improving safety and privacy of patients’ records in sub-Saharan Countries
Arnold M Hamapa, Joseph Mumba Zulu, Oswell Khondowe, Choolwe Jacobs, Mercy Wamunyima Monde, Adam Silumbwe, Lydia Hangulu
Abstract
Background : The healthcare system is increasingly adopting biometric services to deliver safe, efficient, and cost-effective care. However, security concerns arise when users forget their passwords, share them, jot them down, or store them in close proximity to their computers. Further, some passwords are easily guessable, which allows unauthorized access to entire systems. The aim of this systematic review is to appraise evidence on biometric identification strategies for improving safety and privacy of patient’s records in sub-Saharan African Countries
Methods : A systematic review will be conducted on studies that report on medical record security improvement techniques in African countries. Using relevant search terms, a systematic search for suitable peer-reviewed literature will be done in the following electronic bibliographic databases: PubMed, Theses Global and African Journals Online (AJOL), Cochrane Database of Systematic Reviews, and Google Scholar. An electronic search will be carried out for papers describing biometrics in healthcare: improved patient safety and privacy in African countries. The search strategy will encompass biometric terms such as fingerprint, facial, or iris recognition for authentication, along with keywords related to patient safety, Africa, healthcare, and patient privacy.
Discussion : This systematic review will provide a thorough assessment of the existing literature, evidence synthesis, identification of best practices, strategies for overcoming barriers and challenges, consideration of ethical and legal issues, cost-effectiveness assessment, identification of research gaps, and serving as educational resources. The findings will contribute to understanding how biometric play a significant role not only in ensuring the privacy and security of healthcare in sub-Saharan Africa. This review will provide guidance and support evidence-based decision-making in a region with specific healthcare opportunities and challenges by synthesizing current knowledge. A systematic review will direct future efforts and where resources should be allocated in the region by identifying areas that need more research. Overall, the findings of this systematic review.
Before start
Study question
What are the strategies for implementing biometric technologies to improve the safety and privacy of patients’ records in sub-Saharan African healthcare settings and the impact of these strategies on healthcare outcomes within the context of the sub-Saharan African healthcare system?
Steps
Methodology
The reviewers will conduct a systematic review of studies that report on strategies for implementing biometric technologies to improve the safety and privacy of patients’ records in sub-Saharan African healthcare settings and what is the impact of these strategies on healthcare outcomes within the context of the sub-Saharan African healthcare system.
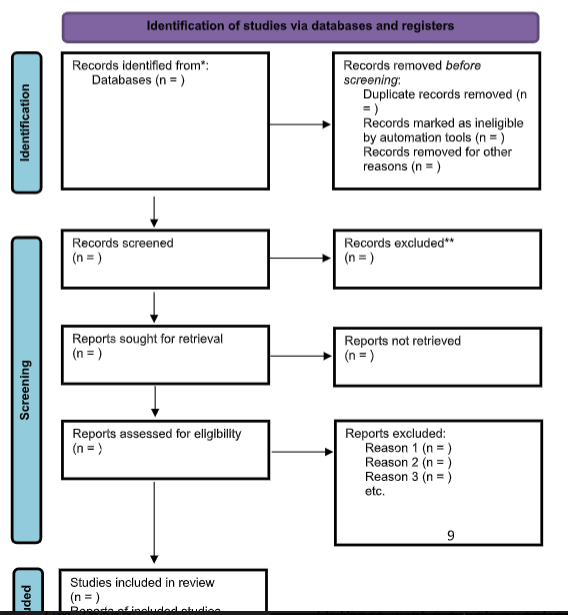
The review will be carried out in accordance with the Preferred Reporting items for Systematic Reviews Protocols (PRISMA-P) (Wells et al., 2000).
The PRISMA 2020 flow diagram for systematic reviews which includes searches of databases and registers will be adapted. This systematic review will follow pre-specified methods as stated in a protocol to be registered in PROSPERO.
The PCC (Population or Participants (P), Concept (C) and Context (C)) framework will guide in identifying the population of interest which are Sub-Saharan African.
Healthcare settings: Concept which is the impact of the strategies on healthcare outcomes: the Context which is sub-Saharan African healthcare system.
Search strategy
A systematic search will be conducted for eligible peer reviewed literature in the following electronic bibliographic databases, PubMed, Theses Global and African Journals online (AJOL), Cochrane Database of Systematic Reviews, Scopus and Google scholar using relevant search terms.
An electronic search will be conducted for studies describing biometrics strategies in healthcare to
improve safety and privacy of patient’s records in sub-Saharan African Countries.
Literature searches will be conducted using a combination of key concepts and Medical Subject Headings (MeSH) with their synonyms, using terms such as biometric (biometrics such as fingerprint, facial or iris recognition as a form of authentication), Patient Safety, Africa, healthcare and privacy for patients.
Reference lists of included studies will be screened for other potential and eligible studies which would have been missed in our searches.
The search strategy will be developed in consultation with a research librarian.
An initial search will be performed in PubMed using the following term combination with appropriate Boolean operators and truncations. Full search terms for the databases are provided as annex: (Biometrics OR "Healthcare" OR "Patient records") AND (Safety OR Privacy) AND "Sub-Saharan Africa" AND "Systematic review" The search strategy will be adapted for each database.
For each search conducted, we will document in detail the date of search, the search engine, and the number of publications retrieved.
Study selection-Inclusion and exclusion criteria
Types of study designs will include randomized controlled trials (RCTs), interventional studies with qualitative, quantitative, and cohort or cross-sectional designs.
Only articles from 2003 to 2023 will be included, as most African countries have implemented national biometric digital identity systems in the past two decades (Digital identification in Africa: Frameworks and initiatives, 2021).
Studies that evaluated biometric programs that cover user-centric, rights- respecting, privacy-respecting, or responsiveness to types of biometrics, policymakers, biometric strategy with regard to identification, surveillance, diagnosis, treatment, follow-up of hospital visitation, patient registration, affecting e-Health, service users, or service providers will be included.
Excluded are non-English papers, observational studies; reviews; abstracts; conference papers and study protocols.
Study selection-Study selection and quality assessment
Two reviewers from a team of five will independently assess titles and abstracts of studies identified by search, using Rayyan screening tool.
The other three reviewers will screen the full texts of identified peer-reviewed articles to evaluate potential eligibility.
Another reviewer’s opinion will be sought in case of persisting disagreements until consensus will be reached.
The study selection will be guided by the PRISMA guidelines, and quality of these studies will be assessed using the critical appraisal skills program (CASP 2015) to ensure methodically proven reliable evidence-based studies in the review.
The following quality criteria will be used:
-
Whether the research questions or objectives were clearly stated?
-
Whether the approach was appropriate for the research question?
-
Whether the study context was clearly described?
-
Whether the role of the researcher was clearly described?
-
Whether the sampling method was clearly described?
-
Whether the sampling strategy was appropriate for the research question?
-
Whether the method of data collection was clearly described?
-
Whether the data collection method was appropriate to the research question?
-
Whether the method of analysis was clearly described?
-
Whether the analysis was appropriate for the research question?
-
Whether the claims made are supported by sufficient evidence?
All studies included in the review will concentrate on Biometrics in healthcare to improve safety and privacy of patient records in sub-Saharan Africa healthcare setting, with clearly stated objectives addressing the review question.
Dealing with missing data
Attempts to contact the authors concerned will be made when important data is missing from the included studies. Missing data will be considered as the absence of any results adding weight to the study and insufficient reporting on study outcomes.
Assessment of reporting biases
Reporting bias will be investigated in every included study depending on the type of study. Each will be assessed by two reviewers for reporting bias. These review authors will discuss any questions or uncertainties that arise during the process.
A third reviewer will be available for any disagreements not resolved.
The studies excluded from the review analysis will not further be assessed at this stage.
The PRISMA guidelines will be used to guide study selection, and the quality of these studies will be assessed using Cochrane Collaboration's Risk of Bias 2 (RoB 2) tool to ensure methodically proven reliable evidence-based studies are included in the review.
The RoB 2 tool will be used to assess the risk of bias in the included RCTs. RoB 2 will be used to assess bias in the following domains: bias arising from the randomization process, bias resulting from deviations from intended interventions, bias resulting from missing outcome data, bias in outcome measurement, bias in the selection of the reported result, and bias in the selection of the reported result. assess publication bias, then synthesize, interpret, and draw conclusions based on the assessment of bias and the synthesis of results, draw conclusions about the efficacy and safety of biometrics-based strategies for improving patient record safety and privacy in Sub-Saharan African countries.
Data extraction and synthesis
To assess information on key study aspects such as objectives, designs, samples, performance measurement tools, and results, data will be extracted into a data extraction form created in Microsoft Excel.
The data extraction form will also include a description of the intervention and outcome measures (Table 1).
The data to be extracted includes author(s) names, date of publication, country of publication study title, journal full reference, aims or research question, participant characteristics, sampling method, study design, data collection, data analysis and most relevant findings conclusions and comments.
Data will be extracted by two independent authors who are AMH and OK who will be responsible for identifying relevant literature and extracting data. Where there will be disagreement, CJ, LH JMZ who are third parties will be consulted. Data from the selected articles will be analyzed using NVivo version 12 software.
Data Analysis
The analysis will involve identification, coding, and exploration of relationships of themes within data.
A code list will be developed which will comprise of broad themes collectively agreed upon by the research team members after preliminary reading of abstracts.
The code list will later be modified to accommodate emergent themes and will be imported into NVivo 12.
Data from the included articles will be coded in the respective nodes by two separate researchers including the principal investigator AMH and OK to allow for inter-coder reliability tests. Where they will be discrepancies, the researchers will discuss until consensus will be reached on how information will be coded.
Code reports will identify specific factors affecting biometrics strategies to improve safety and privacy of patient records in sub-Saharan African healthcare settings.
For any articles where this is unclear, the article will be carried forward into the full-text review.
The full text of the remaining papers will be sourced, and independently evaluated to determine if all
inclusion and exclusion criteria are met.
A descriptive analysis of the contents of all papers reviewed will be conducted per category
(thematic coding) and new (sub) categories deriving from the literature will be added to the framework (Table 2).
Table 2: Summary of included systematic reviews
| A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Country | Study title | Study Population | Sample Size | Strategies | Outcome | Main Findings | Comments/Associated factors |
Strategy for data synthesis
The data from the results of each included study will be extracted into defined data extraction spreadsheets.
The results will be synthesized in a narrative manner.
Based on the included manuscripts in the final library, the plausibility of a meta-analysis will be
assessed depending on the variation of the included data presented.

