Zika NS5 RdRp His-SUMO construct small scale expression and purification protocol
Korvus Wang, michael fairhead, Eleanor Williams
expression
purification
ASAP
CMD
AViDD
Zika
Zika Virus
Zika NS5 NS5 RNA-dependent RNA polymeras...
Zika NS5
Zika RdRp
Zika NS5 RdRp
NS5
RdRp
NS5 RdRp
Disclaimer
Research was supported in part by NIAID of the U.S National Institutes of Health under award number U19AI171399. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The pOPINS-ZVRdRP construct cannot be distributed due to intellectual property restrictions on the vector. We are currently working on re-cloning the sequence into an open access vector, to be available for Addgene orders later.
Abstract
This protocol details the co-expression and purification of Zika NS5 NS5 RNA-dependent RNA polymerase bearing a N-terminal His-SUMO tag at small scale (<6L).
Attachments
Steps
Abbreviations
CV - column volume, total volume of resin in a column
IMAC - immobilised metal affinity chromatography
FT - flow through
LB- Lysogeny Borth
Plasmid Transformation
ZVRdRp N-terminal 6His-SUMO tagged co-expression construct was inoculated from its SixPack glycerol stock.
Protein expression
Scrape off some of the glycerol stock with a sterile loop and use this to inoculate a 50 mL falcon tube containing 10mL of LB supplemented with 50ug/mL carbenicillin. Grow the starter culture at 37°C 4h 0m 0s with 200 rpm shaking.
Use the 10mL starter culture to inoculate 1L auto-induction media (see Materials) supplemented with 50ug/mL carbenicillin in a baffled flask. 200rpm
When the OD600 reaches approximately 2.0, lower the temperature and shaker speed to 180rpm and incubate . Harvest in late afternoon on the second day.
Harvest the cell by centrifugation at 4000x g,4°C. Discard supernatant and store pellet by freezing at -80°C .
Protein Purifcation
Lyse cell pellet
Thaw and resuspend the pellet in ~7mL of lysis buffer per gram of cell pellet. Stir gently with magnetic stir bar at Room temperature for 0h 30m 0s to allow lysozyme and bezonase to start breaking down
cell components.
Lyse by sonication 0h 0m 4s 0h 0m 12s for a total 'on' time of 0h 3m 0s at 40% amplitude to fully rupture the cells. Ensure pellet is °C during sonication to prevent overheating of the sample.
Centrifuge the lysed cells for 38000x g,4°C to remove insoluble cell debris, and collect supernatant in a bottle 4°C
Perform IMAC to extract target protein from the lysed cell mixture
Dispense 1mL Nickel affinity resin Ni Sepharose 6 FF (Cytiva) into a empty gravity flow column. Equilibrate resin by first rinsing with ~ 10CV distilled water, then ~ 10CV binding buffer.
Resuspend the equilibrated resin with some binding buffer and add to the supernatant bottle. Incubate the resin with the supernatant for 0h 10m 0s while rotating or otherwise mixing gently at 4°C
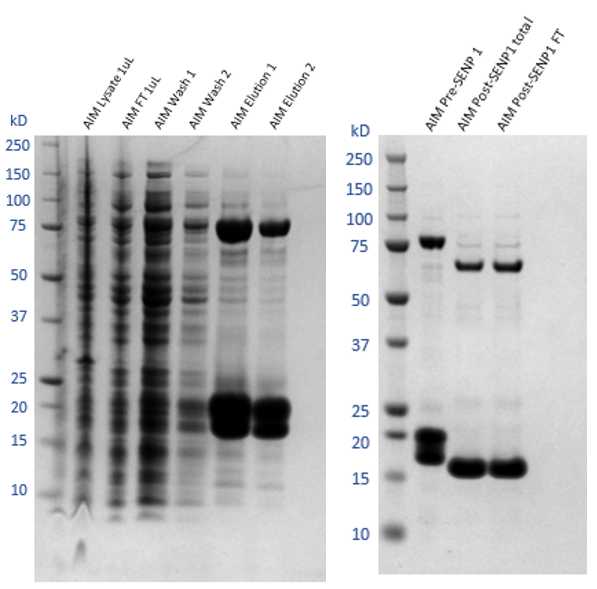
Load the resin/supernatant mix back onto the gravity flow column, retaining the FT separately for SDS-PAGE analysis.
Wash the column with 10CV of base buffer, followed by 10CV of wash buffer twice. Allow wash buffer to pass through completely between washes. This is to remove non-specific and weak binding contaminant proteins from the resin for a cleaner elution.
Collect all the washes separately for SDS-PAGE analysis.
Elute the protein with 5CV of elution buffer.
Repeat step 8.5 one more time, collecting a total of 2 separate elution fractions. This is to ensure maximum retrieval of protein from the resin, as well as removing remaining contaminants for later reverse IMAC.
Measure the absorbance (A280) of the elution fractions using using Nanodrop and estimate the total protein concentration. Although still a mixture of proteins, A280 value can give an estimate of the protein content which will help to determine the amount of protease required to remove the affinity tag.
Wash used IMAC resin with 10CV of base buffer, and leave in the column stored with a small amount of the same buffer such that the resin is kept moist.
This washed IMAC resin will later be reused for reverse IMAC (rIMAC)
Run SDS-PAGE for all samples from total lysis supernatant to final elution. Stain gel with protein staining solution Coomasssie Blue and determine the fractions that contain the protein of interest, by finding the band corresponding to the expected protein molecular weight.
Elution de-salting, tag cleavage and reverse IMAC
Pool and dilute the two elutions with base buffer to 50mL (1:4) , lowering the total imidazole concentration to 60mM.
For tag removal, add His-SENP1 in 1:300 ratio to the total protein content of the diluted sample, as determined by nanodrop. Keep the mixture in the cold room at 4°C
Next day, pass the cleavage mixture over the washed resin (mentioned in step 8.7) three times and collect the final FT.
(Optional) elute rIMAC resin with 2CV elution buffer to confirm if the protein shows non-specific binding to the resin used.
Purify sample further by size exclusion chromatography .
Using 30,000 MWCO spin concentrators, concentrate the fractions, from the rIMAC step, that contain target protein to a maximum final volume of 5mL.
Remove any solid aggregates from the sample by centrifugation at 17200x g,4°C , then immediately draw up the supernatant with a 5mL syringe and a blunt-tip fill needle, taking care not to disturb the pellet.
Using the AKTA Pure system:
Inject the sample onto a 5mL sample loop.
Run the sample down HiLoad 16/60 Superdex 200 pg gel filtration column at 1mL/min in gel filtration buffer, collecting 1mL fractions.
From the chromatogram, analyse fractions F9-H8 by SDS-PAGE.
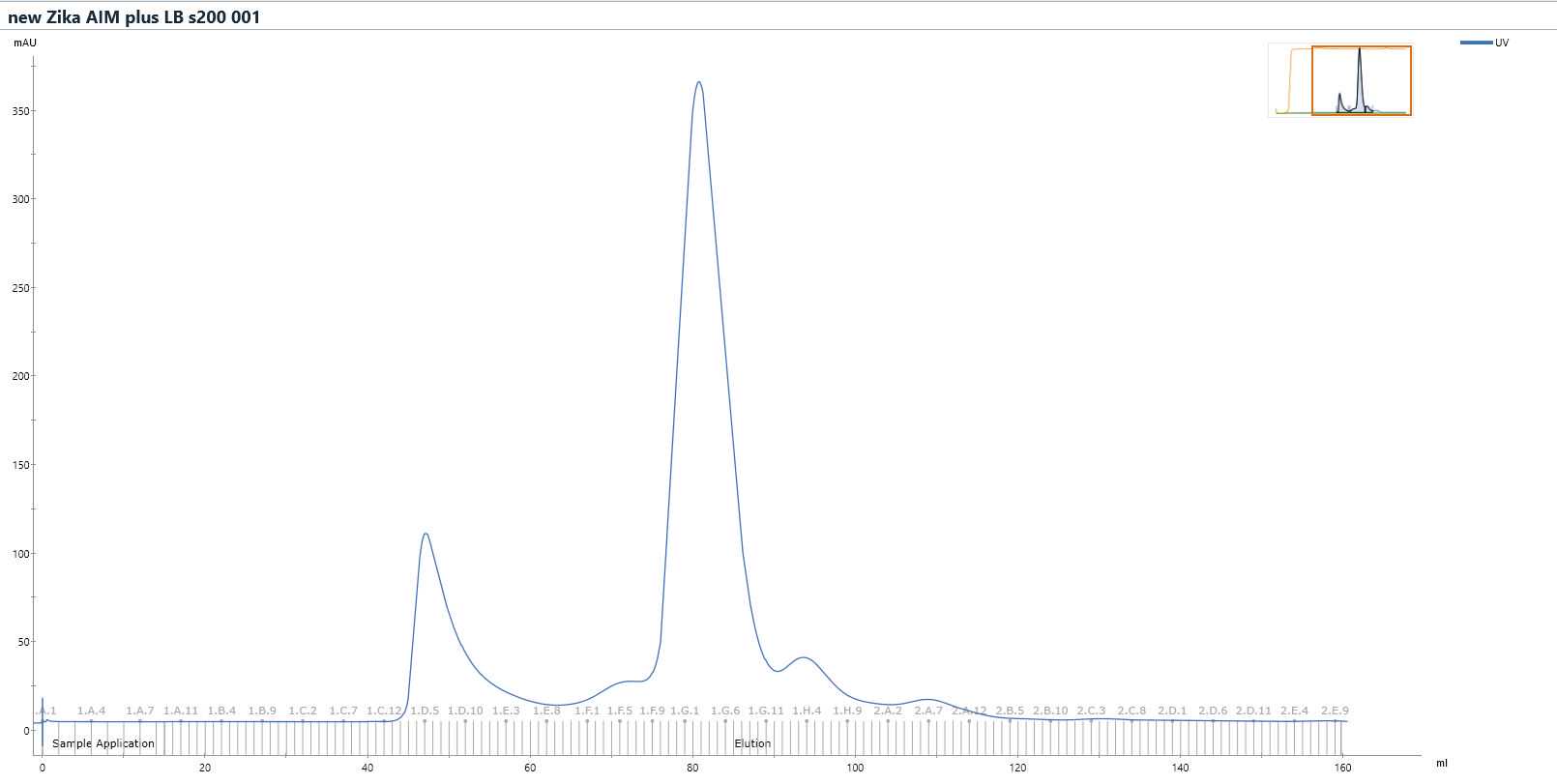
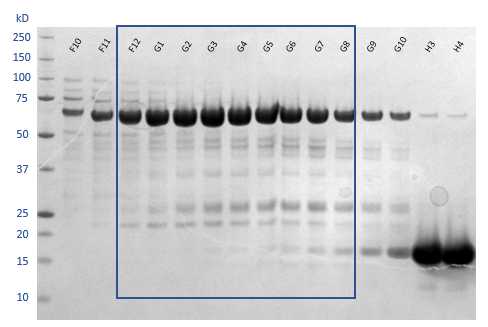
Pool the fractions that contain the target protein, which in this case includes fractions from F12 to G8. Concentrate the sample in Vivaspin 500 30kDa MWCO centrifugal concentrator until the protein concentration reaches4.8mg/mL .
Take 1µL of the final sample for SDS-PAGE.
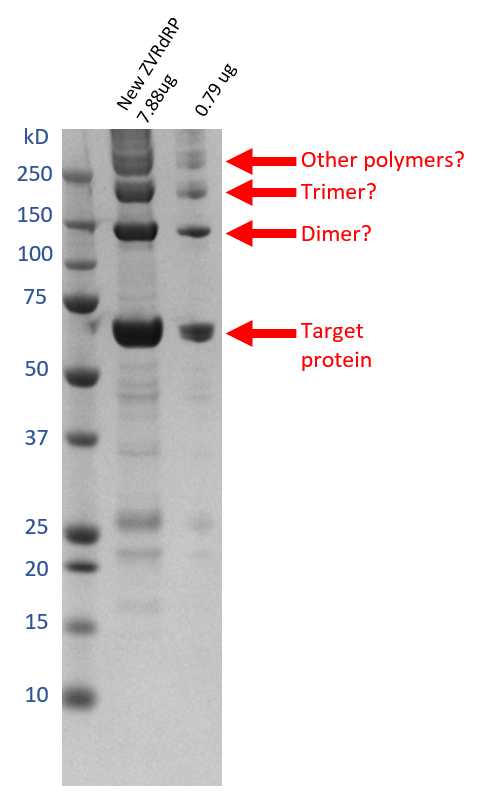
Another 1µL can be taken for mass spectroscopy (MS) analysis, which was not carried out here.
Aliquot into appropriate volumes for future usage to minimise freeze/thaw cycles. Flash-freeze in liquid nitrogen, and store at -80°C until required.