Use of a FlowCam for descriptive measurements of Aureococcus anophagefferens live culture cells
Alex Truchon, Steven W Wilhelm, Emily E. Chase, Laura E Smith
Abstract
A protocol for acquiring an array of descriptive statistics ( e.g., cell count, cell diameter, cell volume) for a monoculture of Aureococcus anophagefferens. This procedure permits an in-depth profile of your cultures via a substantial number of viewable photos and measurements. We recommend this familiarity with your study cells, especially when applying stress treatments such as virus infection, light cycle changes, etc.
Before start
Familiarise yourself with the functions of the FlowCam equipment and Visual Spreadsheet5 software.
Steps
FLOWCAM 8000 OPERATION
An explanation of the operating practices of the Flow CAM 8000 are presented under another protocol and through the relevant software and equipment manuals. Equipment setup can be achieved through the linked protocol below.
SAMPLE AND CONTEXT FILE PREPARATION
Aureococcus anophagefferens cells are retrieved from a culture for measuring. At least 500 μL are required for each sample.
Open the Visual Spreadsheet5 software, and load the appropriate context file. Measuring A. anophagefferens can be accomplished with the following settings in the context file:
Distance to nearest neighbour: 1.0
Close hole iteration: 2.0
Collage image border padding: 2
Dark pixels: 20.0
Light pixels: 20.0
Acceptable region left: 110; right: 1115; top: 1; bottom: 1919
SAMPLE DESCRIPTIVE STATISTICS ACQUISITION
Following set-up of the FlowCam by manufacturer's instructions (using the 20✕ objective, 20✕ flow cells, and 0.5 or 1.0 mL syringe; green set) and the full clearance of Milli-Q water through the tubing, enter the flow cell view mode ( i.e., "Camera View"), apply at least 500 μL of your sample, and start the flow at 1–2 mL/minute. Once cells appear within the on screen camera aim at the flow cell, halt the manual priming (close the window).
Cell image capture (and subsequent descriptive statistics) is processed via the AutoImage mode. Simply click the auto image mode, set the acquisition method constraints, provide a sample name/ID, and initiate the sampling.
Record or export relevant data.
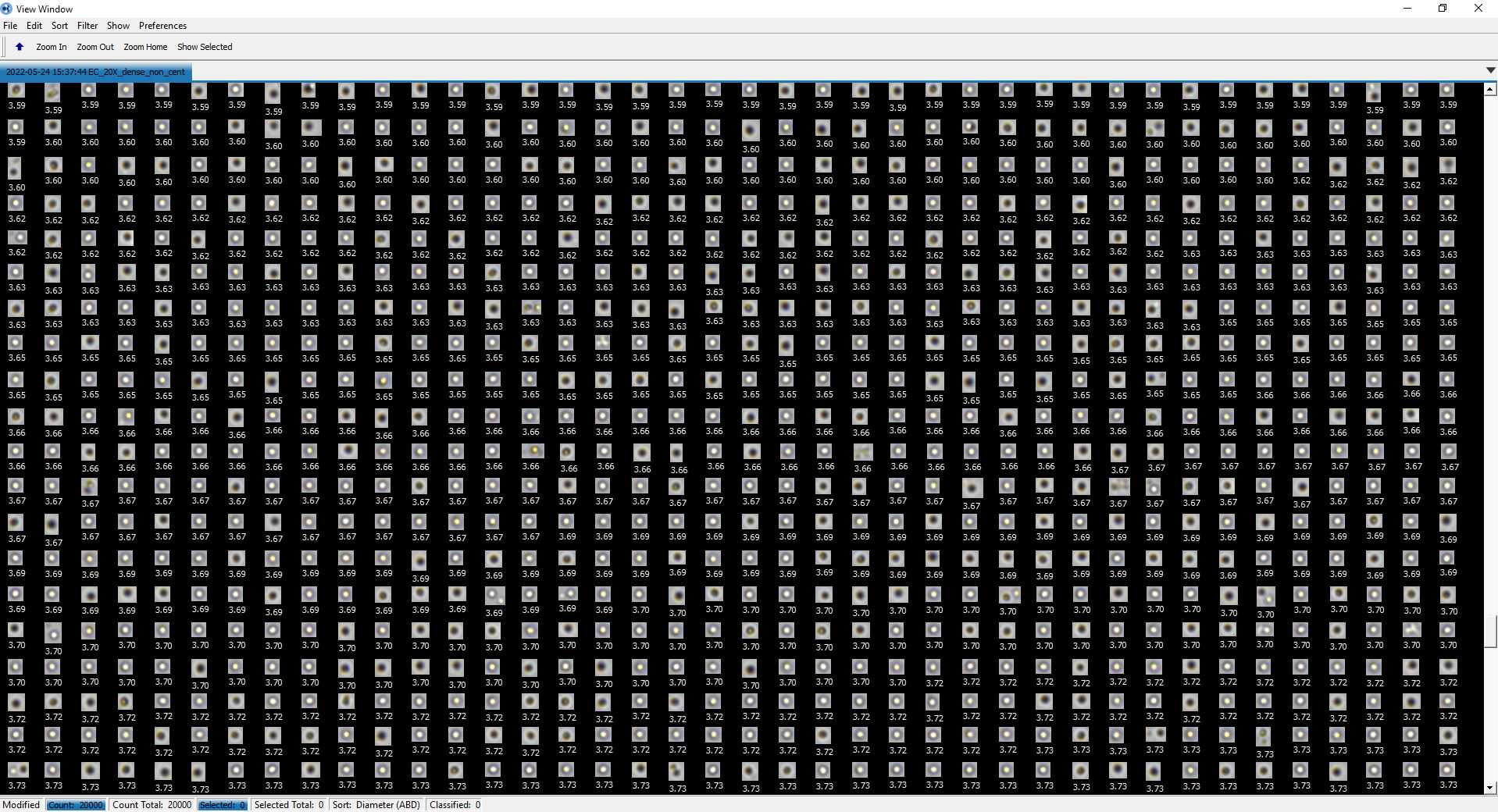
An example of resulting data for A. anophagefferens are provided as Figure 1 and Figure 2 below.
<img src="https://static.yanyin.tech/literature_test/protocol_io_true/protocols.io.14egn2336g5d/j38ibqvyf1.jpg" alt="Figure 1. Aureococcous anophagefferens as defined through the FlowCam AutoImage mode data collection. A histogram defines counts (frequency) of different cell diameters. The Capture X versus Capture Y image explains what position datum (i.e., cells) were measured within the field of the flowcell. A uniform distribution among the Capture X versus Capture Y signifies that the flowcell is unimpeded; a uneven distrubition or "holes" in the collection space would signifiy a block in the flowcell and cause uneven sampling (i.e., photos). " loading="lazy" title="Figure 1. Aureococcous anophagefferens as defined through the FlowCam AutoImage mode data collection. A histogram defines counts (frequency) of different cell diameters. The Capture X versus Capture Y image explains what position datum (i.e., cells) were measured within the field of the flowcell. A uniform distribution among the Capture X versus Capture Y signifies that the flowcell is unimpeded; a uneven distrubition or "holes" in the collection space would signifiy a block in the flowcell and cause uneven sampling (i.e., photos). "/>
Clear the entire FlowCam tubing system of the previous sample, administer a Milli-Q water rinse, and apply a new sample as previously described. Repeat for all samples and then apply FlowCam cleaning as established in your lab.

