Techniques for the detection and counting of starch particles in the atmosphere
Claudio F. Pérez, Soledad Maciel, María Gassmann, Rubén F. Iacono, Mauricio C. De Marzi, Mauro Covi
Abstract
Suspended particles in the atmosphere (aerosols) can impact visibility, the radiation budget, architectural heritage, agricultural activity, and human health (Pérez et al ., 2020). Recent studies suggest that flour derivatives like starch are present in the environment (de Diego et al ., under revision). This research protocol outlines a list of suitable techniques for detecting and recording the presence of starch in the air.
Before start
Carefully wipe all surfaces, materials, and tools with ethanol to avoid any starch contamination during the preparation of samples.
Steps
Preparation of the adhesive mixture and mounting media
Several recipes are in the literature (eg. Käpylä, 1989). But we recommend the following one.
20g
500mL
500mL
60rpm
Weigh 20 g of silicone grease and add to a 1L caramel-colored jar.
Pour the carbon tetrachloride into the jar and gently shake the bottle by opening the lid from time to time to evacuate any vapors that accumulate inside. Let it rest for a day and repeat the shaking operation. Generally, between 24 and 48 hours of this procedure are required until the silicone is completely dissolved. Once the silicone grease has completely dissolved, fill up with 500 mL of acetone.
Carbon tetrachloride can be replaced with 500 mL of acetone, although the silicone solubility is lower and the procedure will require more time.
The preferred mounting media is glycerine with a small amount of phenol to prevent fungal growth. Store in a bottle.
50mL
2mg
Sampling design
This sampling method is based on the volumetric-isokinetic technique designed by Hirst (1952) which is regularly used in Aerobiology.
Equipment
| Value | Label |
|---|---|
| 7 day recording volumetric spore trap | NAME |
| Pollen and spore trap | TYPE |
| Burkard | BRAND |
| 1 | SKU |
The trap (Burkard Manufacturing Co.) filters air at a constant rate of 0.6 m3 per hour. The aerosols are collected on a sticky Melinex plastic tape (provided by the trap manufacturer) that rotates at a steady speed of 2 mm per hour, allowing for the calculation of hourly and daily concentrations (number of particles per m3). The device faces the wind automatically. The tape is replaced weekly ( Fig. 2 ). Find additional details on the operating process in Burkard's manual.

This technique relies on gravity to deposit aerosols suspended in the air. We developed a wooden box trap that prevents the repulsion or attraction of aerosols caused by static charges ( Fig. 2, 3 ). The collected material is supported on a coated slide, and the particles enter through a known size slot. The box trap is positioned horizontally without any surrounding obstacles.

Preparation of adhesive surfaces
Prepare sticky slides spreading the adhesive mixture with a soft brush. The duration of exposure varies according to the research goals.
Preparation of adhesive tape
After placing the Melinex tape on the rotating drum of the Burkard trap (please refer to the trap's user manual for instructions), it should be coated with the adhesive mixture using a soft brush.
Preparation of slides
Add 5 to 6 drops of mounting media (glycerol + phenol) to each exposed slide, cover them with a clean coverslip, and seal them with nail polish.
Preparation of samples
The exposed tape should be cut into 7 pieces, each corresponding to a sampled day, using the Perspex cutting block provided by the trap manufacturer ( Fig. 3, 4, 5 ). All the materials and tools to be used should be carefully cleaned with ethanol.



Detection and counting of starch particles
Samples are studied with an optical microscope with a final magnification of 400 or 1000X according to particle size.
Equipment
| Value | Label |
|---|---|
| Binocular microscope | NAME |
| DM750 | TYPE |
| Leica | BRAND |
| 1 | SKU |
Gravimetric samples require inspection of the total surface of the slide. By performing a simple calculation, we can determine the deposition rates in terms of the number of particles per square centimeter over a specific period. The reference area for this calculation is the area of the wooden slot. We performed one-month exposure periods.
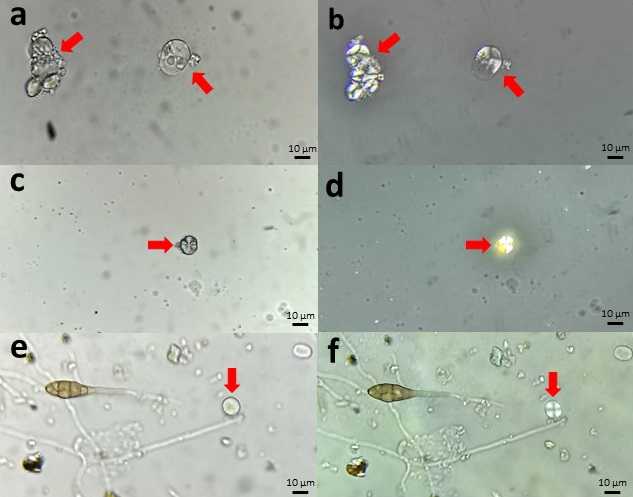
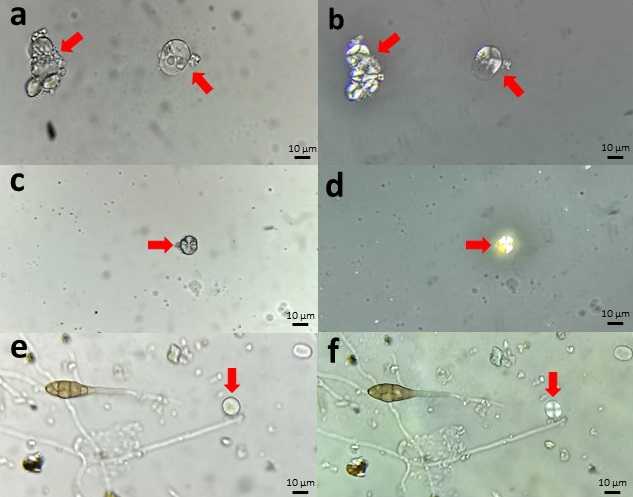
Starch granules appear under the optical microscope as hyaline particles that are difficult to separate from mineral dust like quartz crystals, which are very common in aerosol samples ( Fig. 4 a,c,e ) However, polarized light illumination allows starch to be recognized from a cross-shaped mark ( Fig. 4 b,d,f ) that identifies it among the rest of the aerosols in the sample (Moss, 1976).
Each piece of tape should be glued to a clean slide with the Gelvatol solution ( Fig. 6 ). Add the piece of tape to each slide ( Fig. 7 ).
Preparing the Gelvatol solution
35g
2g
80mL
Add the Gelvatol and the phenol to the water and allow it to stand overnight.
50mL
100
The next day, add the glycerol, heat in a water bath (or warm gradually in a microwave), and stir to mix. Use this to prepare the 10% solution. Store tightly covered. It will last a long time.
10% Gelvatol solution
2mL
18mL
``
Add the 2 ml of Gelvatol solution to the distilled water. Shake, stir, or gently heat in a water bath and mix until the Gelvatol completely disperses. Use this 10% Gelvatol solution to attach the 24-hour segments of exposed Melinex tape onto microscope slides.
Samples are studied with an optical microscope with a final magnification of 400 or 1000X according to particle size.
Equipment
| Value | Label |
|---|---|
| Binocular microscope | NAME |
| DM750 | TYPE |
| Leica | BRAND |
| 1 | SKU |
Volumetric samples can be analyzed at daily or hourly intervals using the standard technique outlined in Käpylä and Penttinen (1981) for aerobiological samples.
Starch granules appear under the optical microscope as hyaline particles that are difficult to separate from mineral dust like quartz crystals, which are very common in aerosol samples ( Fig. 9 a,c,e ) However, polarized light illumination allows starch to be recognized from a cross-shaped mark ( Fig. 9 b,d,f ) that identifies it among the rest of the aerosols in the sample (Moss, 1976).