Targeted analysis of 5-Fluorouracil (5-FU) and Fluoroacetate (FAC) in human plasma by automated PPT+ extraction and LC-HRMS analysis
Natalie ZM Homer, Scott G Denham, Margaux Billen, Joanna Simpson
5-fluorouracil
fluoroacetate
capecitabine metabolism
High Resolution Mass Spectrometry
LC-MS method
ThermoFisher Exploris 240 Orbitrap
Human Plasma
Abstract
This protocol describes the extraction and targeted high-resolution mass spectrometry analysis of two compounds - the anti-cancer drug 5-fluorouracil (5FU) and its catabolite fluoroacetate (FAC), in human plasma samples.
A targeted LC-MS method was developed to measure FAC and 5-FU, by adapting methods from Deenen et al, 2012 and from Leong et al, 2022. Samples were enriched with isotopically labelled lactate and the drug and its metabolite were extracted using automated protein precipitation on an Extrahera liquid handling robot (Biotage) alongside calibration standards.
Analysis of the extract was carried out by liquid chromatography high resolution mass spectrometry (LC-HRMS) in full scan negative mode on an Exploris 240 Orbitrap (ThermoScientific). The amount of each compound (5FU and FAC) in each sample was calculated using linear regression of the peak area ratio of the analytes to the isotopically labelled internal standard.
Analytes

Internal standard

Analyte information
| A | B | C | D |
|---|---|---|---|
| Fluoroacetate | FAC | C2H3FO2 | 78.0117 |
| 5-Fluorouracil | 5-FU | C4H3FN2O2 | 130.0179 |
| 13C3-lactate | 13C3-Lac | [13C]3H6O3 | 93.042 |
Steps
Solvent preparation
Mobile Phase A: H2O + 0.1% Formic Acid 2O + 0.1% Formic Acid
- Add
1Lof LC-MS grade H2O to a 1L glass bottle. - Add
1mLof LC-MS grade Formic Acid to the H2O. - Mix thoroughly.
Mobile Phase B: Acetonitrile
- Add
1Lof LC-MS grade Acetonitrile to a 1L glass bottle.
Autosampler Seal Wash: 10% Acetonitrile
- Add
100mLLC-MS grade Acetonitrile to900mLLC-MS grade H2O in a 1L glass bottle. - Mix thoroughly.
Internal standard preparation
All solutions (internal standards and calibration standards) are kept at -20°C after preparation until use.
Prepare Internal standard stock solution.
13 13C3-Lactate stock solution (1 mg/mL) 3-Lactate stock solution (1 mg/mL)
Using the PS-100 balance, weigh out approximately 2 mg of 13C3-Lactate into a 7 mL glass vial, add appropriate volume of water (HPLC grade) and vortex thoroughly to give a 1 mg/mL 13C3-Lactate stock solution e.g. 2 mL for 2 mg.
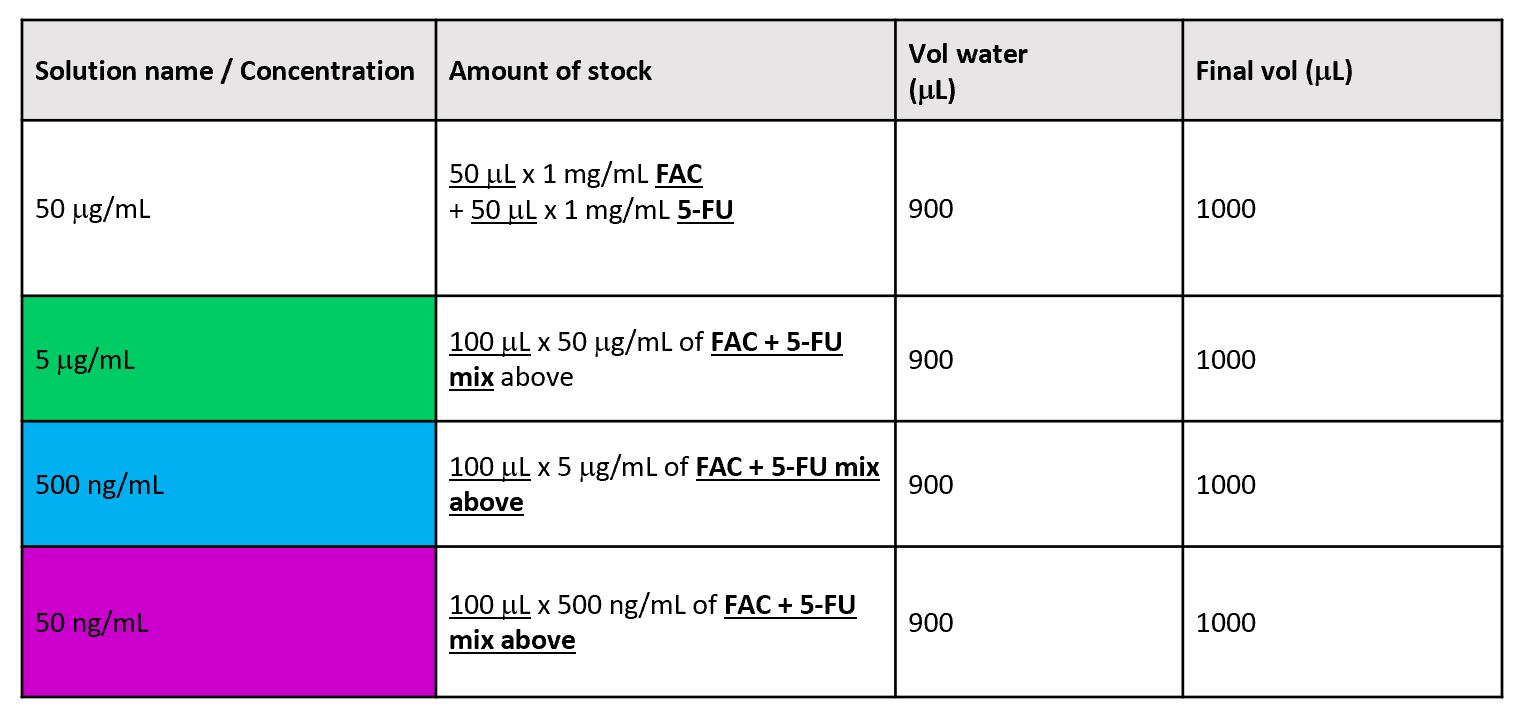
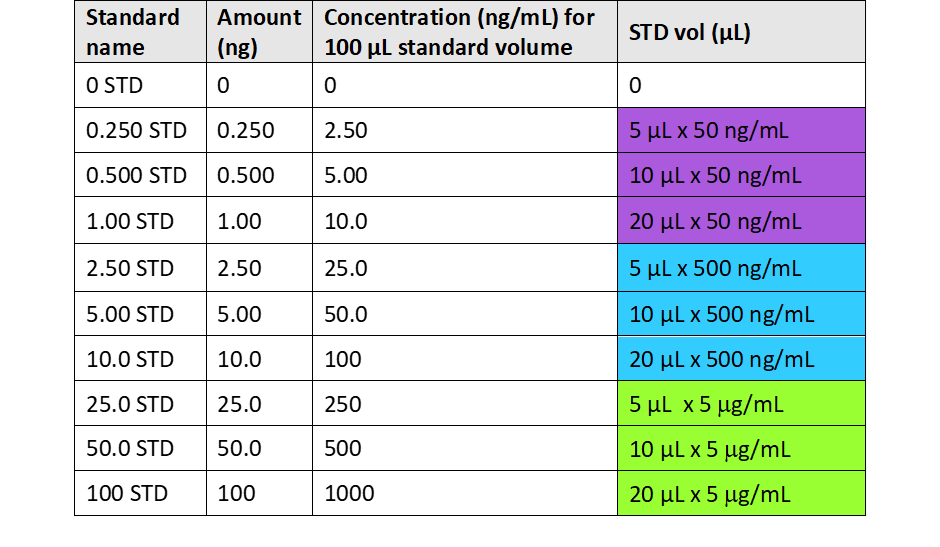
Prepare Internal Standard dilutions according to the table below in:
-
1 x 1.75 mL glass vials labelled '50 µg/mL 13C3-Lactate in water'.
-
1 x 3.5 mL glass vials labelled '5 µg/mL 13C3-Lactate in water'.
| A | B | C | D |
|---|---|---|---|
| 50 mg/mL | 50 µL x 1 mg/mL 13C3-Lactate | 950 | 1000 |
| 5 µg/mL | 200 µL x 50 mg/mL 13C3-Lactate | 1800 | 2000 |
Calibration standards mix preparation
Prepare calibration standard stock solutions.
FAC stock solution (1 mg/mL)
Using a PS-100 balance, weigh out approximately 2 mg of FAC into a 7 mL glass vial, add appropriate volume of water (HPLC grade) and vortex thoroughly to give a 1 mg/mL FAC stock solution e.g. 2 mL for 2 mg.
Store at -20 °C.
5-FU stock solution (1 mg/mL)
Using the PS-100 balance, weigh out approximately 2 mg of 5-FU into a 7 mL glass vial, add appropriate volume of water (HPLC grade) and vortex thoroughly to give a 1 mg/mL 5-FU stock solution e.g. 2 mL for 2 mg.
Store at -20 °C.
Extraction Procedure
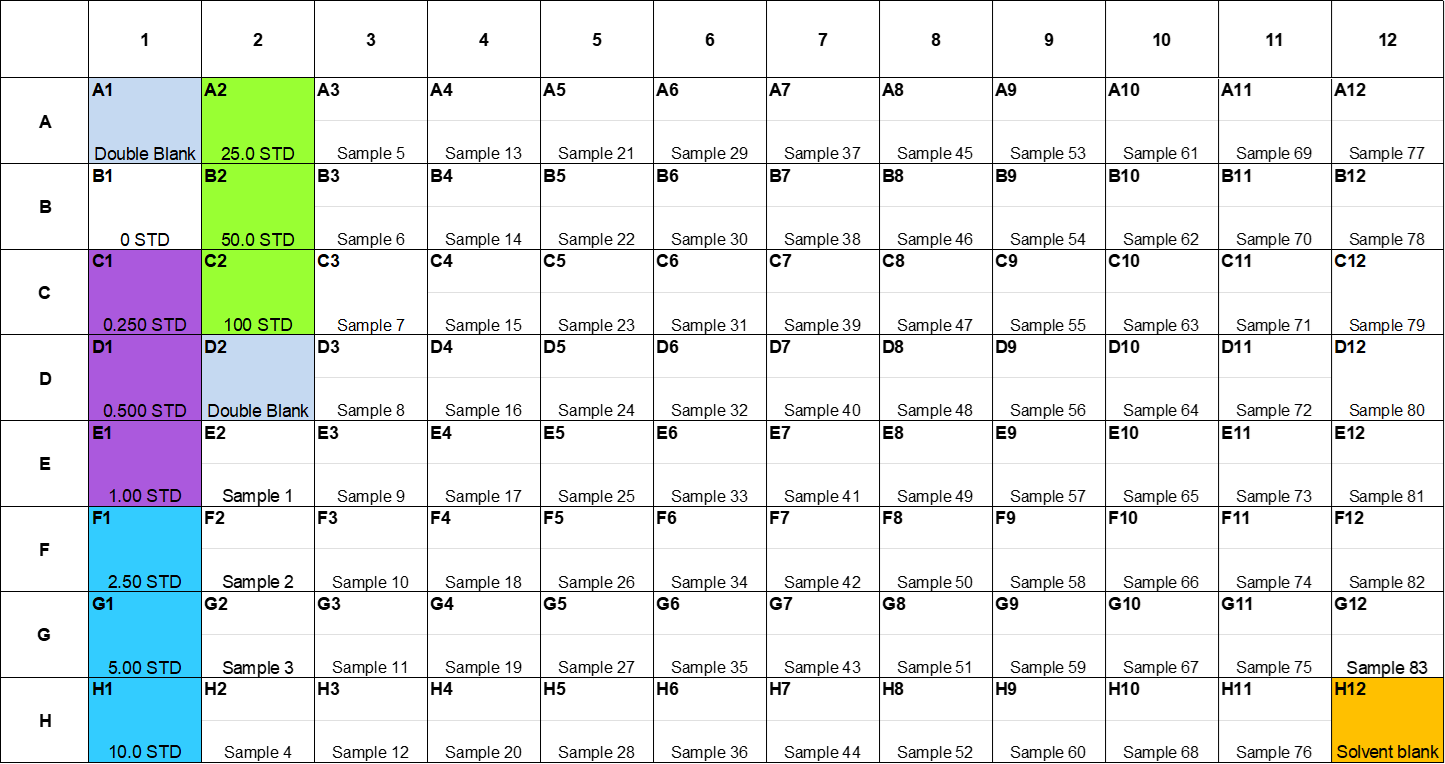
Acquire or build a sample list for the samples in Microsoft Excel including experimental details and unique sample identification codes.
Defrost control pooled human male plasma for calibration standard and blanks preparation.
Defrost calibration standard mixes, internal standards and samples.
Take a 2mL deep well 96 well collection plate (Biotage) and add 100µL control pooled to the calibration standard and blank wells (A1-D2).
Add 100µL of each plasma sample to the appropriate wells.
Add 20µL of the 5 µg/mL internal standard solution to all wells, except for Double Blank and Solvent Blank wells.
Seal the plate using a 96 well plate sealing film (Merck) and shake the plate on a plate shaker for 0h 5m 0s to ensure that the standards and internal standards are sufficiently mixed.
Set up Extrahera liquid handling robot for PPT+ extraction.
Turn on Air Compressor. Make sure a pressure of ~9 bar is achieved and that the compressor goes into Standby (indicated by green flashing light).
Make sure the fume cupboard is switched on and the duct hose is in place in a fume cupboard to ensure proper ventilation.
Turn on Extrahera and wait for it to boot up.
From the Maintenance menu select 'Flush Solvent Inlets' and purge the line which contains LC-MS grade Acetonitrile. Throw away the purged liquid and fill up the container with fresh LC-MS grade Acetonitrile.
Ensure that sufficient number of standard bore solvent tips (deck position 1 and 2) are on the deck.
Place a PPT+ plate in deck position 3. Make sure that it is in the correct orientation and is properly clicked in place.
Take a Waters 2mL 96 well collection plate, and mark it with the project title, investigator name, plate number, extraction date and the initials of the person doing the extraction. Place the plate in carousel position A and make sure that well A1 is on the outside of the carousel next to the A1 label!
Remove the plate seal and place the sample plate on the deck of the Extrahera in position 4.
Select 'Run Single Method' from the Extrahera menu and select an appropriate PPT+ extraction method, then press 'Prepare Run'. Select the columns of the PPT+ plate for processing and update the tip numbers/locations if necessary.
Press Run. The Extrahera loads 300 µL of Acetonitrile into each well. It then transfers the sample plate contents onto the acetonitrile in the PPT+ plate. The Extrahera applies positive pressure to pass the samples through the PPT+ plate and collects the eluent in the Waters 2mL deep well 96 well plate.
Once complete, check the volumes of elution solvent in the collection plate are approximately equal indicating good performance of the positive pressure head. Check that the samples and standards were correctly aspirated from the sample plate.
Place the colection plate on the TurboVap Dual 96 sample concentrator with the gas temperature set to 40 °C and the gas flow to 30 mL/min.
Once dry, resuspend the dry samples in 80µL of H2O and seal the plate with a Waters Adhesive plate sealing film.
Shake the plate for0h 10m 0s at 600 rpm to ensure the samples are resolubilised.
Set up of FAC and 5FU LC-HRMS method and analysis
Put the freshly prepared mobile phases onto the UPLC system. Purge lines with mobile phase A and mobile phase B.
Install an ACQUITY Premier HSS T3 Column 1.8 µm, 2.1 x 150mm column into the column oven. Set the column temperature to 40°C and equilibrate at 100% mobile phase A , 0.3 mL/min for at least 15 minutes. Ensure that the pressure is stable and there are no leaks detectable on the system.
Create an acquisition method in Xcalibur for chromatography and mass spectrometry settings. For chromatography include the following chromatographic gradient conditions in the table below.
Add the detail of the column and mobile phases in the method. Make sure the right column position is selected for the valves and the column oven temperature and column pre-heater are set to 40°C.
| A | B | C | D | E |
|---|---|---|---|---|
| Initial | 0.300 | 100 | 0 | Initial |
| 1.00 | 0.300 | 100 | 0 | 5 |
| 2.80 | 0.300 | 5 | 95 | 5 |
| 3.50 | 0.300 | 5 | 95 | 5 |
| 3.60 | 0.300 | 100 | 0 | 5 |
| 7.00 | 0.300 | 100 | 0 | 5 |
Chromatographic gradient for separation of FAC and 5-FU in extracted plasma samples on an ACQUITY Premier HSS T3 Column 1.8 µm, 2.1 x 150mm using H2O + 0.1% Formic Acid (Mobile Phase A) and Acetonitrile (Mobile Phase B).
Add the following mass spectrometry method parameters to the acquisition method:
| A | B |
|---|---|
| Instrument | Thermo Exploris 240 Orbitrap |
| Source, Ionisation Mode | Thermo Scientific™ OptaMax™ NG ion source (H-ESI) |
| Scan Mode, Polarity | Full Scan, Negative |
| Mass range | 50 - 200 m/z |
| Resolution | 120 000 |
| Acquisition time | 7.0 min |
| Sheath Gas | 50 |
| Aux Gas | 15 |
| Sweep Gas | 1 |
| IonSpray Voltage (IS) (Negative) | -2500 V |
| Ion Transfer Tube Temperature | 350°C |
| Vaporizer Temperature | 450°C |
| Probe position (x – axis) | 2 |
| Probe position (y – axis) | 2 |
Place the sealed 96-well plate into the autosampler of the chromatography system.
Create a batch in Xcalibur - use the correct position for the 96-well plate, the correct position of the column, the correct lines for the mobile phases and the correct LC-MS/MS method. Name and save the Batch acquisition file. Use the same naming convention to name the resulting data file .
Set volume of injection to 20 µL and submit batch to analyse.
Test the system with a mid-standard curve point injection and then complete the batch in order from A1 to H12.
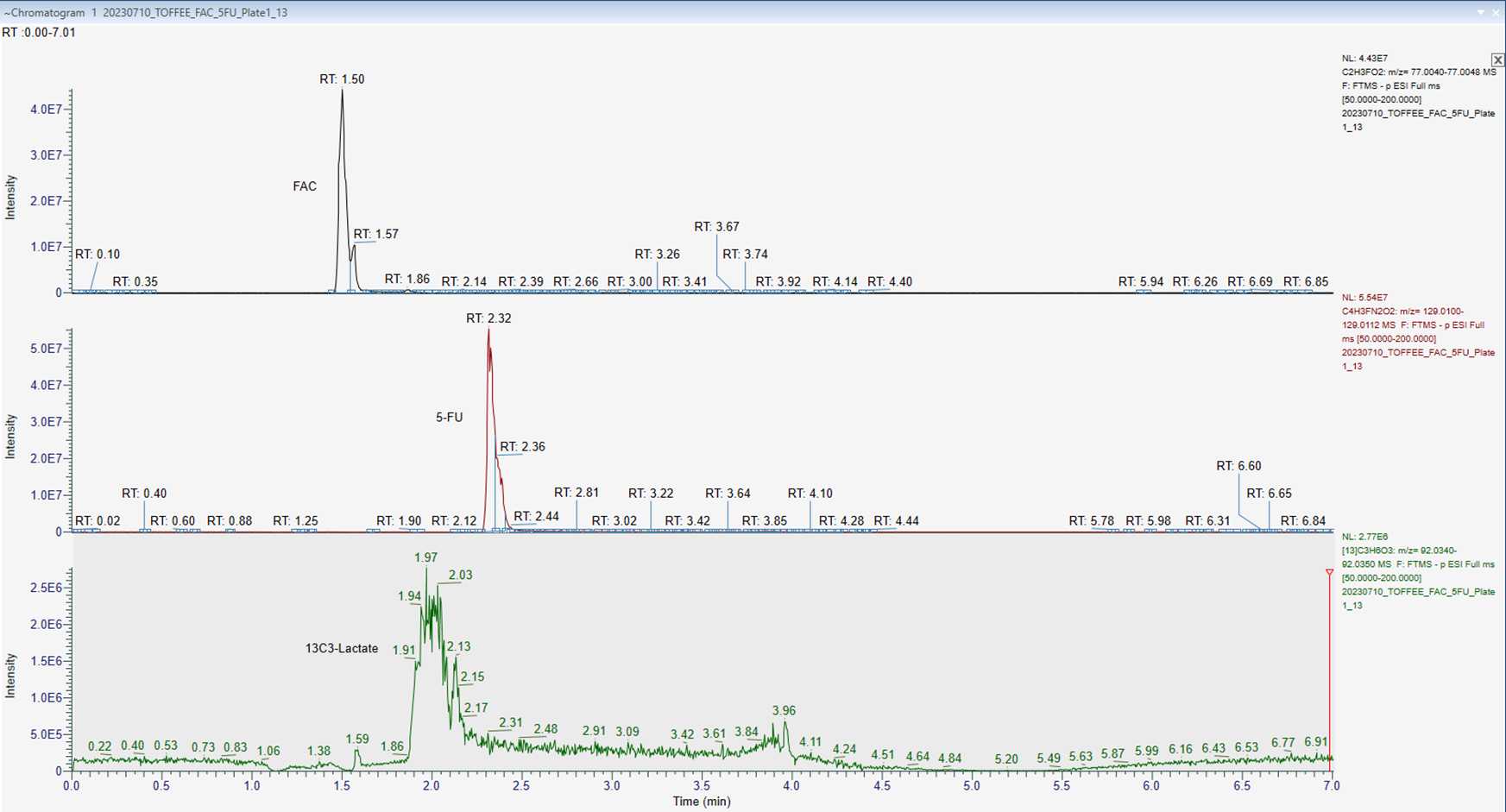
Use the m/z [M-H]- for each compound in the table below to interrogate the data:
| A | B | C | D |
|---|---|---|---|
| m/z [M-H]- | 77.0044 | 129.0106 | 92.0345 |
| Retention time (min) | 1.50 | 2.32 | 1.97 |
Example chromatography of FAC, 5-FU and 13C3-Lactate separation is shown below. Separation performed on an ACQUITY Premier HSS T3 Column 1.8 µm, 2.1 x 150mm using a system of H2O + 0.1% Formic Acid (Mobile Phase A) and Acetonitrile (Mobile Phase B).
Data Analysis using TraceFinder
Use the protocol below and the compound specific table details (mass and retention time) in Step 32 to evaluate the data and obtain the FAC and 5FU concentrations in the samples analysed:
Margaux Billen, Scott G Denham, Joanna P Simpson, Natalie ZM Homer 2023. Using TraceFinder and Excel software to evaluate and report multi-analyte targeted LC-MS data acquired on an ThermoScientific Exploris 240 Orbitrap. protocols.io https://dx.doi.org/10.17504/protocols.io.n92ldm8z7l5b/v1